Ocular hypertension, Open-angle glaucoma
Adult: As 0.005% solution: Instil 1 drop into the affected eye(s) once daily, preferably in the evening.
|
Indications and Dosage
Ophthalmic
Ocular hypertension, Open-angle glaucoma Adult: As 0.005% solution: Instil 1 drop into the affected eye(s) once daily, preferably in the evening.
|
|
Incompatibility
Precipitation may occur when eye drops containing thiomersal are mixed with latanoprost.
|
|
Contraindications
Active intraocular inflammation, active herpes simplex keratitis; history of recurrent herpetic keratitis associated with prostaglandin analogues.
|
|
Special Precautions
Patient with history of herpes simplex keratitis; aphakia, pseudophakia with torn posterior lens capsule or anterior chamber lenses; risk factors for cystoid macular oedema, predisposition for or history of intraocular inflammation (e.g. iritis or uveitis); chronic angle closure glaucoma, pigmentary glaucoma, congenital glaucoma, inflammatory glaucoma, neovascular glaucoma, acute attacks of closed-angle glaucoma; asthma. Use during peri-operative period of cataract surgery. Pregnancy and lactation.
|
|
Adverse Reactions
Significant: Reactivation of herpes simplex keratitis; intraocular inflammation, macular oedema (including cystoid macular oedema); increased brown pigmentation of iris and eyelid skin; eyelash and vellus hair changes (e.g. increase in number, length, thickness and pigmentation; misdirected growth of eyelashes); bacterial keratitis (when multiple-dose container used is inadvertently contaminated).
Eye disorders: Mild to moderate conjunctival hyperaemia, eye irritation (e.g. itching, grittiness, burning, stinging and foreign body sensation); punctate keratitis (mostly without symptoms), blepharitis, conjunctivitis; eyelid oedema, localised skin reaction on the eyelids, photophobia, blurred vision, eye pain. Musculoskeletal and connective tissue disorders: Myalgia, arthralgia. Nervous system disorders: Dizziness, headache. Respiratory, thoracic and mediastinal disorders: Dyspnoea. Rarely, exacerbation of asthma. Skin and subcutaneous tissue disorders: Rash. |
|
Ophth: C
|
|
Patient Counseling Information
This drug may cause transient blurring of vision, if affected, do not drive or operate machinery. Remove contact lenses prior to administration and reinsert them after 15 minutes.
|
|
Monitoring Parameters
Monitor IOP. Regularly assess patients who develop noticeably increased iris pigmentation.
|
|
Overdosage
Symptoms: Ocular irritation and conjunctival hyperaemia. Management: Symptomatic treatment.
|
|
Drug Interactions
May cause paradoxical elevations in IOP when used concomitantly with prostaglandins or other prostaglandin analogues or derivatives.
|
|
Action
Description:
Mechanism of Action: Latanoprost, an isopropyl ester prodrug, is a prostaglandin F2α analogue and a selective prostanoid agonist. Its exact mechanism of action has not been fully expounded; however, it is suggested that it reduces intraocular pressure (IOP) by increasing the outflow of aqueous humour through the uveoscleral pathway. Onset: Approx 3-4 hours. Duration: At least 24 hours. Pharmacokinetics: Absorption: Well absorbed through the cornea. Time to peak plasma concentration: Approx 2 hours. Distribution: Volume of distribution: 0.16 ± 0.02 L/kg. Metabolism: Rapidly and completely hydrolysed in the cornea by esterases into latanoprost acid (biologically active); systemically absorbed latanoprost acid is mainly metabolised in the liver via fatty acid β-oxidation into 1,2-dinor and 1,2,3,4-tetranor metabolites. Excretion: Via urine (88%; as metabolites). Elimination half-life: 17 minutes. |
|
Chemical Structure
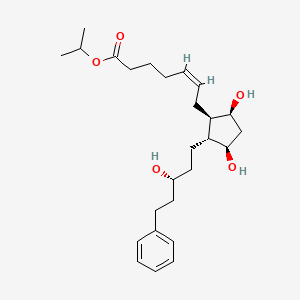 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 5311221, Latanoprost. https://pubchem.ncbi.nlm.nih.gov/compound/Latanoprost. Accessed Oct. 25, 2023. |
|
Storage
Unopened bottle: Store between 2-8°C. Do not freeze. Protect from light. Opened bottle: Store below 25°C and use within 4 weeks. Protect from light.
|
|
MIMS Class
|
|
ATC Classification
S01EE01 - latanoprost ; Belongs to the class of prostaglandin analogues. Used in the treatment of glaucoma.
|
|
References
Anon. Latanoprost. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 23/06/2023. Anon. Latanoprost. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 23/06/2023. Buckingham R (ed). Latanoprost. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 23/06/2023. Joint Formulary Committee. Latanoprost. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 23/06/2023. Latanoprost 50 micrograms/mL Eye Drops Solution (Kent Pharma UK Limited). MHRA. https://products.mhra.gov.uk. Accessed 23/06/2023. OptaProst Ophthalmic Solution 0.005% w/v (Pharmaniaga Marketing Sdn Bhd). National Pharmaceutical Regulatory Agency - Ministry of Health Malaysia. https://www.npra.gov.my. Accessed 23/06/2023. Teva Pharma (New Zealand) Limited. Latanoprost (Teva), 50 micrograms/mL, Eye Drops data sheet 04 November 2022. Medsafe. http://www.medsafe.govt.nz. Accessed 23/06/2023. Xalatan Solution (Pfizer Laboratories Div Pfizer Inc). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed. Accessed 23/06/2023.
|