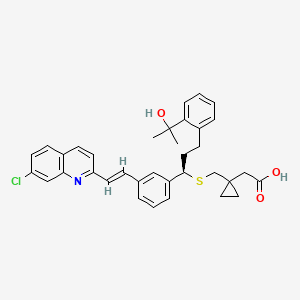
Perennial allergic rhinitis
Adult: As conventional tab: 10 mg once daily.
Child: 6-23 months As granules: 1 sachet (4 mg) once daily in the evening; 2-5 years As 4 mg chewable tab or granules: 1 tab or sachet once daily in the evening; 6-14 years As 5 mg chewable tab: 1 tab once daily in the evening; ≥15 years As conventional tab: Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: 6-23 months As granules: 1 sachet (4 mg) once daily in the evening; 2-5 years As 4 mg chewable tab or granules: 1 tab or sachet once daily in the evening; 6-14 years As 5 mg chewable tab: 1 tab once daily in the evening; ≥15 years As conventional tab: Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Seasonal allergic rhinitis
Adult: As conventional tab: 10 mg once daily.
Child: 2-5 years As 4 mg chewable tab or granules: 1 tab or sachet once daily in the evening; 6-14 years As 5 mg chewable tab: 1 tab once daily in the evening; ≥15 years As conventional tab: Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: 2-5 years As 4 mg chewable tab or granules: 1 tab or sachet once daily in the evening; 6-14 years As 5 mg chewable tab: 1 tab once daily in the evening; ≥15 years As conventional tab: Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Chronic asthma
Adult: As conventional tab: 10 mg once daily in the evening.
Child: 6-23 months As granules: 1 sachet (4 mg) once daily in the evening; 2-5 years As 4 mg chewable tab or granules: 1 tab or sachet once daily in the evening; 6-14 years As 5 mg chewable tab: 1 tab once daily in the evening; ≥15 years As conventional tab: Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: 6-23 months As granules: 1 sachet (4 mg) once daily in the evening; 2-5 years As 4 mg chewable tab or granules: 1 tab or sachet once daily in the evening; 6-14 years As 5 mg chewable tab: 1 tab once daily in the evening; ≥15 years As conventional tab: Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Prophylaxis of exercise-induced asthma
Adult: As conventional tab: 10 mg given at least 2 hours before exercise; limit to 1 dose every 24 hours.
Child: 2-5 years As 4 mg chewable tab or granules: 1 tab or sachet once daily in the evening; 6-14 years As 5 mg chewable tab: 1 tab once daily in the evening; ≥15 years As conventional tab: Same as adult dose.
Child: 2-5 years As 4 mg chewable tab or granules: 1 tab or sachet once daily in the evening; 6-14 years As 5 mg chewable tab: 1 tab once daily in the evening; ≥15 years As conventional tab: Same as adult dose.




 Sign Out
Sign Out