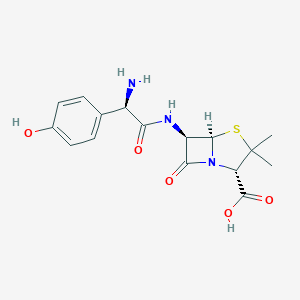
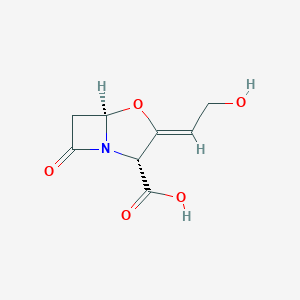
Prophylaxis of surgical infections
Adult: Available preparations:
Amoxicillin 500 mg and clavulanic acid 100 mg powder for solution for inj or infusion
Amoxicillin 1,000 mg and clavulanic acid 200 mg powder for solution for inj or infusion
In major surgical procedures involving the head and neck, cardiac, gastrointestinal tract, biliary tract, renal, joint replacement, and pelvic cavity: For procedures <1 hour duration: 1,000 mg/200 mg to 2,000 mg/200 mg given at induction of anaesthesia; For procedures >1 hour duration: 1,000 mg/200 mg to 2,000 mg/200 mg given at induction of anaesthesia, then up to 3 more 1,000 mg/500 mg doses in 24 hours. Administer normal IV or oral treatment course post-operatively, if clear signs of infection at operation is observed.
Amoxicillin 500 mg and clavulanic acid 100 mg powder for solution for inj or infusion
Amoxicillin 1,000 mg and clavulanic acid 200 mg powder for solution for inj or infusion
In major surgical procedures involving the head and neck, cardiac, gastrointestinal tract, biliary tract, renal, joint replacement, and pelvic cavity: For procedures <1 hour duration: 1,000 mg/200 mg to 2,000 mg/200 mg given at induction of anaesthesia; For procedures >1 hour duration: 1,000 mg/200 mg to 2,000 mg/200 mg given at induction of anaesthesia, then up to 3 more 1,000 mg/500 mg doses in 24 hours. Administer normal IV or oral treatment course post-operatively, if clear signs of infection at operation is observed.
Intravenous
Acute exacerbations of chronic bronchitis, Bone and joint infections, Community-acquired pneumonia, Cystitis, Ear, nose and/or throat infections, Genitourinary infections, Infected animal bites, Intra-abdominal infections, Lower respiratory tract infections, Osteomyelitis, Pyelonephritis, Skin and soft tissue infections, Upper respiratory tract infections
Adult: Available preparations:
Amoxicillin 500 mg and clavulanic acid 100 mg powder for solution for inj or infusion
Amoxicillin 1,000 mg and clavulanic acid 200 mg powder for solution for inj or infusion
Individualise dosing based on expected susceptible pathogens, severity and site of infection, age, weight and renal function of the patient. 1,000 mg/200 mg 8 hourly via slow inj over 3-4 minutes or via drip or infusion over 30-40 minutes. In more serious infections, frequency may be increased to 6 hourly intervals. Treatment duration: Up to 14 days without review; osteomyelitis may require longer periods of treatment. Consider local guidelines on appropriate dosing frequencies and recommendations.
Child: Available preparations:
Amoxicillin 500 mg and clavulanic acid 100 mg powder for solution for inj or infusion
Amoxicillin 1,000 mg and clavulanic acid 200 mg powder for solution for inj or infusion
<3 months or weighing <4 kg: 25 mg/5 mg/kg 12 hourly via infusion only over 30-40 minutes; ≥3 months <40 kg: 25 mg/5 mg/kg 8 hourly via slow IV inj over 3-4 minutes or via drip or infusion over 30-40 minutes; ≥40 kg: Same as adult dose.
Amoxicillin 500 mg and clavulanic acid 100 mg powder for solution for inj or infusion
Amoxicillin 1,000 mg and clavulanic acid 200 mg powder for solution for inj or infusion
Individualise dosing based on expected susceptible pathogens, severity and site of infection, age, weight and renal function of the patient. 1,000 mg/200 mg 8 hourly via slow inj over 3-4 minutes or via drip or infusion over 30-40 minutes. In more serious infections, frequency may be increased to 6 hourly intervals. Treatment duration: Up to 14 days without review; osteomyelitis may require longer periods of treatment. Consider local guidelines on appropriate dosing frequencies and recommendations.
Child: Available preparations:
Amoxicillin 500 mg and clavulanic acid 100 mg powder for solution for inj or infusion
Amoxicillin 1,000 mg and clavulanic acid 200 mg powder for solution for inj or infusion
<3 months or weighing <4 kg: 25 mg/5 mg/kg 12 hourly via infusion only over 30-40 minutes; ≥3 months <40 kg: 25 mg/5 mg/kg 8 hourly via slow IV inj over 3-4 minutes or via drip or infusion over 30-40 minutes; ≥40 kg: Same as adult dose.
Oral
Acute bacterial sinusitis, Cystitis, Ear, nose and/or throat infections, Genitourinary infections, Infected animal bites, Intra-abdominal infections, Pyelonephritis, Skin and soft tissue infections, Upper respiratory tract infections
Adult: Available preparations:
Amoxicillin 250 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 500 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 875 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 1,000 mg and clavulanic acid 62.5 mg extended-release tab
Amoxicillin 125 mg and clavulanic acid 31.25 mg per 5 mL oral susp
Amoxicillin 250 mg and clavulanic acid 62.5 mg per 5 mL oral susp
Individualise dosing based on expected susceptible pathogens, severity and site of infection, age, weight and renal function of the patient. As conventional tab: 250 mg/125 mg tid, or 500 mg/125 mg tid, or 875 mg/125 mg bid. As oral susp: 500 mg/125 mg tid. Treatment duration: Up to 14 days. without review. For treatment of acute bacterial sinusitis only: As extended-release tab: 2,000 mg/125 mg 12 hourly for 10 days.
Child: Available preparations:
Amoxicillin 125 mg and clavulanic acid 31.25 mg per 5 mL oral susp
Amoxicillin 250 mg and clavulanic acid 62.5 mg per 5 mL oral susp
≤6 years <40 kg: 20 mg/5 mg/kg daily to 60 mg/15 mg/kg daily given in 3 divided doses; ≥40 kg: Same as adult dose.
Amoxicillin 400 mg and clavulanic acid 57 mg per 5 mL oral susp
≤6 years <40 kg: 25 mg/3.6 mg/kg daily to 45 mg/6.4 mg/kg daily given in 2 divided doses. For sinusitis: Up to 70 mg/10 mg/kg daily may be given in 2 divided doses.
Amoxicillin 250 mg and clavulanic acid 125 mg conventional tab
≥40 kg: Same as adult dose.
Amoxicillin 500 mg and clavulanic acid 125 mg conventional tab
25-<40 kg: 20 mg/5 mg/kg daily to 60 mg/15 mg/kg daily given in 3 divided doses; ≥40 kg: Same as adult dose.
Amoxicillin 1,000 mg and clavulanic acid 62.5 mg extended-release tab
For treatment of acute bacterial sinusitis only: ≥40 kg: Same as adult dose.
Amoxicillin 250 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 500 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 875 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 1,000 mg and clavulanic acid 62.5 mg extended-release tab
Amoxicillin 125 mg and clavulanic acid 31.25 mg per 5 mL oral susp
Amoxicillin 250 mg and clavulanic acid 62.5 mg per 5 mL oral susp
Individualise dosing based on expected susceptible pathogens, severity and site of infection, age, weight and renal function of the patient. As conventional tab: 250 mg/125 mg tid, or 500 mg/125 mg tid, or 875 mg/125 mg bid. As oral susp: 500 mg/125 mg tid. Treatment duration: Up to 14 days. without review. For treatment of acute bacterial sinusitis only: As extended-release tab: 2,000 mg/125 mg 12 hourly for 10 days.
Child: Available preparations:
Amoxicillin 125 mg and clavulanic acid 31.25 mg per 5 mL oral susp
Amoxicillin 250 mg and clavulanic acid 62.5 mg per 5 mL oral susp
≤6 years <40 kg: 20 mg/5 mg/kg daily to 60 mg/15 mg/kg daily given in 3 divided doses; ≥40 kg: Same as adult dose.
Amoxicillin 400 mg and clavulanic acid 57 mg per 5 mL oral susp
≤6 years <40 kg: 25 mg/3.6 mg/kg daily to 45 mg/6.4 mg/kg daily given in 2 divided doses. For sinusitis: Up to 70 mg/10 mg/kg daily may be given in 2 divided doses.
Amoxicillin 250 mg and clavulanic acid 125 mg conventional tab
≥40 kg: Same as adult dose.
Amoxicillin 500 mg and clavulanic acid 125 mg conventional tab
25-<40 kg: 20 mg/5 mg/kg daily to 60 mg/15 mg/kg daily given in 3 divided doses; ≥40 kg: Same as adult dose.
Amoxicillin 1,000 mg and clavulanic acid 62.5 mg extended-release tab
For treatment of acute bacterial sinusitis only: ≥40 kg: Same as adult dose.
Oral
Acute exacerbations of chronic bronchitis, Acute otitis media, Bone and joint infections, Community-acquired pneumonia, Lower respiratory tract infections, Osteomyelitis
Adult: Available preparations:
Amoxicillin 500 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 875 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 1,000 mg and clavulanic acid 62.5 mg extended-release tab
Amoxicillin 125 mg and clavulanic acid 31.25 mg per 5 mL oral susp
Amoxicillin 250 mg and clavulanic acid 62.5 mg per 5 mL oral susp
Individualise dosing based on expected susceptible pathogens, severity and site of infection, age, weight and renal function of the patient. As conventional tab: 500 mg/125 mg tid, or 875 mg/125 mg 2 or 3 times daily. As oral susp: 500 mg/125 mg tid. Treatment duration: Up to 14 days without review; osteomyelitis may require longer periods of treatment. For treatment of community-acquired pneumonia only: As extended-release tab: 2,000 mg/125 mg 12 hourly for 7-10 days.
Child: Available preparations:
Amoxicillin 125 mg and clavulanic acid 31.25 mg per 5 mL oral susp
Amoxicillin 250 mg and clavulanic acid 62.5 mg per 5 mL oral susp
≤6 years <40 kg: 20 mg/5 mg/kg daily to 60 mg/15 mg/kg daily given in 3 divided doses; ≥40 kg: Same as adult dose.
Amoxicillin 400 mg and clavulanic acid 57 mg per 5 mL oral susp
≤6 years <40 kg: 25 mg/3.6 mg/kg daily to 45 mg/6.4 mg/kg daily given in 2 divided doses. For otitis media and lower respiratory tract infections: Up to 70 mg/10 mg/kg daily may be given in 2 divided doses.
Amoxicillin 500 mg and clavulanic acid 125 mg conventional tab
25-<40 kg: 20 mg/5 mg/kg daily to 60 mg/15 mg/kg daily given in 3 divided doses; ≥40 kg: Same as adult dose.
Amoxicillin 1,000 mg and clavulanic acid 62.5 mg extended-release tab
For treatment of community-acquired pneumonia only: ≥40 kg: Same as adult dose.
Amoxicillin 500 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 875 mg and clavulanic acid 125 mg conventional tab
Amoxicillin 1,000 mg and clavulanic acid 62.5 mg extended-release tab
Amoxicillin 125 mg and clavulanic acid 31.25 mg per 5 mL oral susp
Amoxicillin 250 mg and clavulanic acid 62.5 mg per 5 mL oral susp
Individualise dosing based on expected susceptible pathogens, severity and site of infection, age, weight and renal function of the patient. As conventional tab: 500 mg/125 mg tid, or 875 mg/125 mg 2 or 3 times daily. As oral susp: 500 mg/125 mg tid. Treatment duration: Up to 14 days without review; osteomyelitis may require longer periods of treatment. For treatment of community-acquired pneumonia only: As extended-release tab: 2,000 mg/125 mg 12 hourly for 7-10 days.
Child: Available preparations:
Amoxicillin 125 mg and clavulanic acid 31.25 mg per 5 mL oral susp
Amoxicillin 250 mg and clavulanic acid 62.5 mg per 5 mL oral susp
≤6 years <40 kg: 20 mg/5 mg/kg daily to 60 mg/15 mg/kg daily given in 3 divided doses; ≥40 kg: Same as adult dose.
Amoxicillin 400 mg and clavulanic acid 57 mg per 5 mL oral susp
≤6 years <40 kg: 25 mg/3.6 mg/kg daily to 45 mg/6.4 mg/kg daily given in 2 divided doses. For otitis media and lower respiratory tract infections: Up to 70 mg/10 mg/kg daily may be given in 2 divided doses.
Amoxicillin 500 mg and clavulanic acid 125 mg conventional tab
25-<40 kg: 20 mg/5 mg/kg daily to 60 mg/15 mg/kg daily given in 3 divided doses; ≥40 kg: Same as adult dose.
Amoxicillin 1,000 mg and clavulanic acid 62.5 mg extended-release tab
For treatment of community-acquired pneumonia only: ≥40 kg: Same as adult dose.




 Sign Out
Sign Out