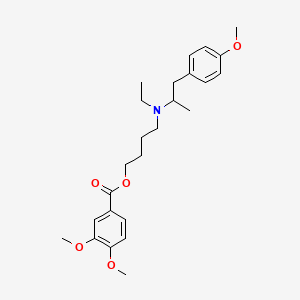
Gastrointestinal spasm, Irritable bowel syndrome
Adult: As conventional tab: 135 mg tid. As oral susp: 150 mg tid. As modified-release cap: 200 mg bid. Dose may be gradually reduced after several weeks when the desired effect has been obtained. Treatment recommendations may vary between individual products or among countries (refer to specific product guidelines).
Child: ≥10 years As oral susp: 150 mg tid. Dose may be gradually reduced after several weeks when the desired effect has been obtained. Treatment recommendations may vary between individual products or among countries (refer to specific product guidelines).
Child: ≥10 years As oral susp: 150 mg tid. Dose may be gradually reduced after several weeks when the desired effect has been obtained. Treatment recommendations may vary between individual products or among countries (refer to specific product guidelines).




 Sign Out
Sign Out