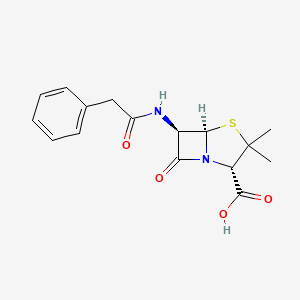
Brain abscess, Gas gangrene, Leptospirosis, Listeriosis, Osteomyelitis, Pasteurella infection, Respiratory tract infections, Septicaemia, Skin infections
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. Dosage: 600-3,600 mg (1-6 million units) daily in 4-6 divided doses via IV inj, IV infusion, or IM inj. Higher doses (up to 14,400 mg [24 million units] daily) may be needed for more serious infections and are given via IV, often by infusion. Rate of not more than 300 mg/min is recommended for IV doses exceeding 1,200 mg (2 million units). Dosage and treatment duration depend on the pathogen type and susceptibility, severity of infection, and patient condition. Treatment recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: 1 month to 12 years 18-60 mg/kg (30,000-100,000 units/kg) daily in 4-6 divided doses via IV inj, IV infusion, or IM inj. High doses of 60-300 mg/kg (100,000-500,000 units/kg) daily in 4-6 divided doses may be given via IV. Dosage and treatment duration depend on the pathogen type and susceptibility, severity of infection, and patient condition. Treatment recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: 1 month to 12 years 18-60 mg/kg (30,000-100,000 units/kg) daily in 4-6 divided doses via IV inj, IV infusion, or IM inj. High doses of 60-300 mg/kg (100,000-500,000 units/kg) daily in 4-6 divided doses may be given via IV. Dosage and treatment duration depend on the pathogen type and susceptibility, severity of infection, and patient condition. Treatment recommendations may vary among countries and individual products (refer to specific product guidelines).
Intramuscular, Intravenous
Diphtheria
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. As adjunctive treatment after antitoxin administration and for prevention of carrier state: 1,200-1,800 mg (2-3 million units) daily in divided doses 4-6 hourly. Doses may be given via IV infusion or IM inj; IV administration is advisable when large doses are needed. Treatment duration: 10-12 days. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: As adjunctive treatment after antitoxin administration and for prevention of carrier state: 90-150 mg/kg (150,000-250,000 units/kg) daily in divided doses 6 hourly. Doses may be given via IV infusion or IM inj. Treatment duration: 7-10 days. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: As adjunctive treatment after antitoxin administration and for prevention of carrier state: 90-150 mg/kg (150,000-250,000 units/kg) daily in divided doses 6 hourly. Doses may be given via IV infusion or IM inj. Treatment duration: 7-10 days. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Intramuscular, Intravenous
Actinomycosis
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. For cervicofacial disease: 600-3,600 mg (1-6 million units) daily in divided doses 4-6 hourly. For thoracic and abdominal disease: 6,000-12,000 mg (10-20 million units) daily in divided doses 4-6 hourly. Doses may be given via IV infusion or IM inj; IV administration is advisable when large doses are needed. Treatment recommendations may vary among countries and individual products (refer to specific product guidelines).
Intramuscular, Intravenous
Suspected meningococcal disease
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. Dosage: 1,200 mg (2 million units) as a single dose via IV or IM inj prior to transfer to hospital.
Child: 1-11 months 300 mg (500,000 units); 1-9 years 600 mg (1 million units); 10-17 years 1,200 mg (2 million units). All doses are given as a single dose via IV or IM inj prior to transfer to hospital.
Child: 1-11 months 300 mg (500,000 units); 1-9 years 600 mg (1 million units); 10-17 years 1,200 mg (2 million units). All doses are given as a single dose via IV or IM inj prior to transfer to hospital.
Intramuscular, Intravenous
Botulism
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. As adjunctive therapy to antitoxin: 12,000 mg (20 million units) daily in divided doses 4-6 hourly. Doses may be given via IV infusion or IM inj; IV administration is advisable when large doses are needed. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Intramuscular, Intravenous
Rat bite fever
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. For cases caused by susceptible Spirillum minus or Streptobacillus moniliformis: 7,200-12,000 mg (12-20 million units) daily in divided doses 4-6 hourly for 3-4 weeks. Doses may be given via IV infusion or IM inj; IV administration is advisable when large doses are needed. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Intramuscular, Intravenous
Tetanus
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. As an alternative adjunctive treatment to tetanus Ig: 12,000 mg (20 million units) daily in divided doses 4-6 hourly for 7-10 days. Doses may be given via IV infusion or IM inj; IV administration is advisable when large doses are needed. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Intravenous
Bacterial endocarditis
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. Dosage: 7,200-14,400 mg (12-24 million units) daily in divided doses 4-6 hourly via IV infusion. Dosage and treatment duration depend on the pathogen type and susceptibility. Recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: 120-180 mg/kg (200,000-300,000 units/kg) daily in divided doses 4 hourly via IV infusion for at least 4 weeks. Max: 14,400 mg (24 million units) daily. For some resistant organisms, may use in combination with gentamicin. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: 120-180 mg/kg (200,000-300,000 units/kg) daily in divided doses 4 hourly via IV infusion for at least 4 weeks. Max: 14,400 mg (24 million units) daily. For some resistant organisms, may use in combination with gentamicin. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Intravenous
Intrapartum prophylaxis against group B streptococcal infection in neonates
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. Initially, 3,000 mg (5 million units) as a single loading dose given to the mother, followed by 1,500 mg (2.5 million units) 4 hourly until delivery. Doses are given via IV inj or infusion.
Intravenous
Bacterial meningitis
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. Dosage: 2,400 mg (4 million units) 4 hourly via IV inj or infusion. Treatment duration may vary depending on the causative pathogen and clinical response. Dosage or treatment recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: 180-240 mg/kg (300,000-400,000 units/kg) daily in divided doses 4-6 hourly. Max: 2,400 mg (4 million units) per dose; 14,400 mg (24 million units) daily. Doses are given via IV infusion. Dosage or treatment recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: 180-240 mg/kg (300,000-400,000 units/kg) daily in divided doses 4-6 hourly. Max: 2,400 mg (4 million units) per dose; 14,400 mg (24 million units) daily. Doses are given via IV infusion. Dosage or treatment recommendations may vary among countries and individual products (refer to specific product guidelines).
Intravenous
Anthrax
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. Dosage: 4,800 mg (8 million units) daily in divided doses 6 hourly via IV infusion. Higher doses may be needed depending on pathogen susceptibility. Concomitant use with other antibacterials may also be indicated. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: As part of appropriate combination regimen: 240 mg/kg (400,000 units/kg) daily in divided doses 4 hourly. Max: 2,400 mg (4 million units) per dose. Doses are given via IV infusion. Treatment duration: At least 14 days if meningitis is excluded or at least 14-21 days if meningitis cannot be excluded, and until the patient is clinically stable. Recommendations may vary among countries (refer to local guidelines).
Child: As part of appropriate combination regimen: 240 mg/kg (400,000 units/kg) daily in divided doses 4 hourly. Max: 2,400 mg (4 million units) per dose. Doses are given via IV infusion. Treatment duration: At least 14 days if meningitis is excluded or at least 14-21 days if meningitis cannot be excluded, and until the patient is clinically stable. Recommendations may vary among countries (refer to local guidelines).
Intravenous
Neurosyphilis
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. Dosage: 1,200-2,400 mg (2-4 million units) 4 hourly via IV infusion for 10-14 days.
Child: ≥1 month 30 mg/kg (50,000 units/kg) 4-6 hourly for 10-14 days. Max: 14,400 mg (24 million units) daily. Doses are given via IV infusion.
Child: ≥1 month 30 mg/kg (50,000 units/kg) 4-6 hourly for 10-14 days. Max: 14,400 mg (24 million units) daily. Doses are given via IV infusion.
Intravenous
Lyme disease
Adult: Benzylpenicillin 600 mg is equivalent to approx 1 million international units. As an alternative agent: 10,800-14,400 mg (18-24 million units) daily in divided doses 4 hourly. Treatment duration: 10-28 days (early stage); 14-28 days (late stage). Alternatively, 12,000-18,000 mg (20-30 million units) daily in 2-3 divided doses for 14 days. Doses are given via IV infusion. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: As an alternative agent: 120-240 mg/kg (200,000-400,000 units/kg) daily in divided doses 4 hourly. Max: 14,400 mg (24 million units) daily. Alternatively, 300 mg/kg (500,000 units/kg) daily in 2-3 divided doses. Doses are given via IV infusion. Treatment duration depends on the clinical syndrome. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: As an alternative agent: 120-240 mg/kg (200,000-400,000 units/kg) daily in divided doses 4 hourly. Max: 14,400 mg (24 million units) daily. Alternatively, 300 mg/kg (500,000 units/kg) daily in 2-3 divided doses. Doses are given via IV infusion. Treatment duration depends on the clinical syndrome. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).




 Sign Out
Sign Out