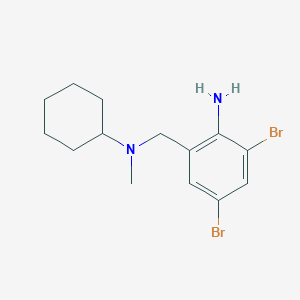
Mucolytic
Adult: For the treatment of bronchopulmonary diseases or conditions associated with excessive mucous secretion: 8 mg tid as needed, may be increased to 16 mg tid for the 1st 7 days.
Child: 2-5 years 2 mg tid or 4 mg bid (Max: 8 mg daily); 6-11 years 4-8 mg tid (Max: 24 mg daily); ≥12 years Same as adult dose. Treatment or dosage recommendations may vary among countries and individual products (refer to detailed product guideline).
Child: 2-5 years 2 mg tid or 4 mg bid (Max: 8 mg daily); 6-11 years 4-8 mg tid (Max: 24 mg daily); ≥12 years Same as adult dose. Treatment or dosage recommendations may vary among countries and individual products (refer to detailed product guideline).




 Đăng xuất
Đăng xuất