Ocular hypertension, Open-angle glaucoma
Adult: As omidenepag isopropyl: Instil 1 drop into the affected eye(s) once daily in the evening.
|
Indications and Dosage
Ophthalmic
Ocular hypertension, Open-angle glaucoma Adult: As omidenepag isopropyl: Instil 1 drop into the affected eye(s) once daily in the evening.
|
|
Contraindications
Aphakia or pseudophakia. Concomitant use with tafluprost.
|
|
Special Precautions
Patient with active ocular inflammation (e.g. iritis, uveitis), angle-closure glaucoma. Pregnancy and lactation.
|
|
Adverse Reactions
Significant: Increased pigmentation of the iris and eyelid skin, eyelash changes (e.g. increased length, thickness, and number of lashes), ocular inflammation, macular oedema (including cystoid macular oedema).
Eye disorders: Conjunctival hyperaemia, photophobia, blurred vision, dry eye, eye pain or irritation, ocular hyperaemia, punctate keratitis, visual impairment, corneal thickening, corneal epithelial disorders. General disorders and administration site conditions: Instillation site pain. Nervous system disorders: Headache. |
|
Patient Counseling Information
This drug may cause temporary blurred vision or photophobia, if affected, do not drive or operate machinery. If you are using more than 1 topical eye medicine, administer them at least 5 minutes apart. Remove contact lenses prior to application and reinsert 15 minutes after administration.
|
|
Monitoring Parameters
Monitor IOP. Regularly examine patients who develop increased iris pigmentation.
|
|
Drug Interactions
Increased risk of moderate to severe photophobia and ocular inflammation (e.g. iritis) with tafluprost. Increased frequency of ocular inflammatory adverse reactions (e.g. conjunctival hyperaemia) with timolol.
|
|
Action
Description:
Mechanism of Action: Omidenepag is a relatively selective prostaglandin E2 (EP2) receptor agonist which decreases IOP. The exact mechanism of its IOP-lowering effect is currently unknown; however, it is considered to increase aqueous outflow via both trabecular and uveoscleral outflow pathways by stimulating EP2 receptors. Pharmacokinetics: Absorption: Omidenepag isopropyl (prodrug) is absorbed through the cornea. Time to peak plasma concentration: 10-15 minutes. Metabolism: Omidenepag isopropyl is rapidly metabolised in the eye to omidenepag via hydrolysis by carboxylesterase-1; omidenepag is further metabolised in the liver via oxidation, N-dealkylation, glucuronidation, and sulfate or taurine conjugation. |
|
Chemical Structure
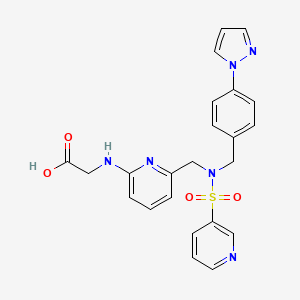 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 44230575, Omidenepag. https://pubchem.ncbi.nlm.nih.gov/compound/Omidenepag. Accessed Mar. 29, 2023. |
|
Storage
Unopened bottle: Store between 2-8°C. Once opened, it can be stored up to 30°C and use within 1 month. Protect from light.
|
|
MIMS Class
|
|
ATC Classification
S01EX06 - omidenepag ; Belongs to the class of other antiglaucoma preparations.
|
|
References
Anon. Omidenepag Isopropyl. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 26/01/2023. Anon. Omidenepag Isopropyl. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 27/01/2023. Eybelis Ophthalmic Solution 0.002% (Santen Pharma Malaysia Sdn. Bhd.). MIMS Malaysia. http://www.mims.com/malaysia. Accessed 26/01/2023. Eybelis Ophthalmic Solution 0.002% (Santen Pharma Malaysia Sdn. Bhd.). National Pharmaceutical Regulatory Agency - Ministry of Health Malaysia. https://www.npra.gov.my. Accessed 26/01/2023. Eybelis Ophthalmic Solution 0.002% (Santen Pharmaceutical Co., Ltd.). MIMS Singapore. http://www.mims.com/singapore. Accessed 26/01/2023. Omlonti Ophthalmic Solution 0.002% (Santen Incorporated). U.S. FDA. https://www.fda.gov. Accessed 26/01/2023. Omlonti Solution/Drops (Santen Incorporated). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed. Accessed 26/01/2023.
|