HIV-1 infection
Adult: In combination with ritonavir in treatment-experienced patients, resistant to other protease inhibitors: 500 mg bid.
|
Indications and Dosage
Oral
HIV-1 infection Adult: In combination with ritonavir in treatment-experienced patients, resistant to other protease inhibitors: 500 mg bid.
|
|
Hepatic Impairment
Moderate to severe (Child-Pugh class B or C): Contraindicated.
|
|
Administration
Should be taken with food.
|
|
Contraindications
Hypersensitivity. Moderate to severe hepatic impairment (Child-Pugh class B or C). Lactation. Concomitant administration with drugs that highly dependent on CYP3A for clearance or potent CYP3A inducers.
|
|
Special Precautions
Patient with diabetes mellitus, haemophilia A or B, risk factor for increased bleeding (e.g. trauma, surgery, other pathological conditions); sulfonamide allergy. Mild hepatic impairment (including chronic hepatitis B or C). Pregnancy.
|
|
Adverse Reactions
Significant: Fat redistribution (e.g. buffalo hump, breast enlargement, central obesity, cushingoid appearance, facial wasting, peripheral wasting), hyperglycaemia or diabetes mellitus, hyperlipidaemia, immune reconstitution syndrome, skin reaction (e.g. urticarial rash, maculopapular rash, photosensitivity).
Gastrointestinal disorders: Abdominal distention, abdominal pain, diarrhoea, dyspepsia, flatulence, nausea, vomiting. General disorders and admin site conditions: Fatigue. Metabolism and nutrition disorders: Hypertriglyceridaemia. Nervous system disorders: Headache. Potentially Fatal: Hepatotoxicity (e.g. hepatitis, hepatic failure), intracranial haemorrhage. |
|
Monitoring Parameters
Monitor total cholesterol and triglyceride at baseline and during therapy; LFT at baseline and frequently during therapy; viral load, CD4, glucose as clinically indicated. Monitor for signs and symptoms of hepatitis/hepatotoxicity.
|
|
Drug Interactions
Increased serum concentration with fluconazole. May increase serum concentration of bosentan, fluticasone, midazolam (IV), rifabutin, trazodone, atorvastatin. Increased risk of QT prolongation, palpitations, sinus tachycardia with salmeterol. Decreased serum concentration with anticonvulsants (e.g. carbamazepine, phenobarbital, phenytoin). May decrease serum concentration of ethinyl estradiol, nucleoside reverse transcriptase inhibitors (e.g. abacavir, didanosine, zidovudine), protease inhibitors (e.g. amprenavir, lopinavir, saquinavir), dolutegravir, meperidine. May produce disulfiram-like reaction with metronidazole.
Potentially Fatal: May increase plasma concentration and increase risk of serious adverse events of drugs highly dependent on CYP3A for clearance or potent CYP3A inducers (e.g. alfuzosin; antiarrhythmics [e.g. amiodarone, bepridil, flecainide, propafenone, quinidine]; ergot derivatives [e.g. dihydroergotamine, ergonovine, ergotamine, methylergonovine]; rifampicin, cisapride; HMG-CoA reductase inhibitors [e.g. lovastatin, simvastatin]; antipsychotics (e.g. pimozide, lurasidone]; sildenafil; sedative/hypnotic [e.g. oral midazolam, triazolam]. |
|
Food Interaction
Decreased plasma concentration with St. John's wort, avoid use.
|
|
Action
Description: Tipranavir is a non-peptide protease inhibitor which binds to HIV-1 protease activity site and prevents cleavage of viral Gag-Pol polyprotein precursors into individual functional proteins. Thus, resulting in the formation of immature, non-infectious viral particles.
Pharmacokinetics: Absorption: Limited absorption after oral administration. Time to peak plasma concentration: Approx 1-5 hours. Distribution: Crosses the placenta. Volume of distribution: 7.7-10.2 L. Plasma protein binding: >99.9% to albumin and α1- acid glycoprotein. Metabolism: Metabolised in the liver mainly by CYP3A4. Excretion: Mainly via faeces (82.3%); urine (4.4 %). Elimination half-life: 5.5 hours (females); 6 hours (males). |
|
Chemical Structure
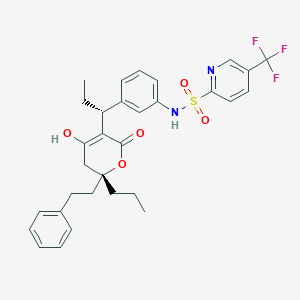 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 54682461, Tipranavir. https://pubchem.ncbi.nlm.nih.gov/compound/Tipranavir. Accessed Dec. 21, 2020. |
|
Storage
Cap: Store between 2-8°C. Do not freeze. Oral solution: Store between 15-25°C. Do not refrigerate or freeze.
|
|
MIMS Class
|
|
ATC Classification
J05AE09 - tipranavir ; Belongs to the class of protease inhibitors. Used in the systemic treatment of viral infections.
|
|
References
Anon. Tipranavir. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 21/10/2020. Anon. Tipranavir. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 21/10/2020. Aptivus Capsules, Liquid Filled, Solution (Boehringer Ingelheim Pharmaceuticals, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 23/10/2020. Buckingham R (ed). Tipranavir. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 21/10/2020. Joint Formulary Committee. Tipranavir. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 21/10/2020.
|