Conjunctival decongestant
Adult: As 0.05% tetryzoline hydrochloride solution: Instill 1-2 drops into the affected eye(s) 4 times daily.
|
Indications and Dosage
Ophthalmic
Conjunctival decongestant Adult: As 0.05% tetryzoline hydrochloride solution: Instill 1-2 drops into the affected eye(s) 4 times daily.
|
|
Special Precautions
Patient with CV disease (e.g. hypertension, coronary artery disease), diabetes, narrow-angle glaucoma, hyperthyroidism. Pregnancy and lactation.
|
|
Adverse Reactions
Cardiac disorders: Palpitations, tachycardia.
Eye disorders: Blurred vision, eye irritation, mydriasis, rebound congestion (prolonged use). General disorders and administration site conditions: Weakness, sweating. Nervous system disorders: Headache, tremor. Vascular disorders: Hypertension. |
|
Patient Counseling Information
Remove contact lenses prior to ophthalmic administration and reinsert after 10 minutes.
|
|
Action
Description: Tetryzoline is an α-adrenergic receptor agonist. The exact mechanism of action has not been fully elucidated, but it is believed to cause vasoconstriction by stimulating arterioles of the conjunctiva.
Synonym: tetrahydrozoline. Onset: Within few minutes. Duration: 4-8 hours. |
|
Chemical Structure
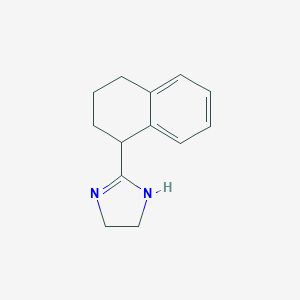 Source: National Center for Biotechnology Information. PubChem Database. Tetrahydrozoline, CID=5419, https://pubchem.ncbi.nlm.nih.gov/compound/Tetrahydrozoline (accessed on Jan. 23, 2020) |
|
Storage
Store between 15-25°C.
|
|
MIMS Class
|
|
ATC Classification
S01GA02 - tetryzoline ; Belongs to the class of sympathomimetics used as ophthalmologic decongestants.
|
|
References
Anon. Tetrahydrozoline (Ophthalmic). Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 10/04/2018. Anon. Tetrahydrozoline. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com/. Accessed 10/04/2018. Buckingham R (ed). Tetryzoline Hydrochloride. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 10/04/2018. CVS Redness Relief Original Solution/drops (CVS Pharmacy, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 12/04/2018. Visine Original Redness Relief Solution/drops (Johnson & Johnson Consumer Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 10/04/2018.
|