Osteoporosis in postmenopausal women, Prophylaxis of postmenopausal osteoporosis
Adult: 60 mg once daily.
|
Indications and Dosage
Oral
Osteoporosis in postmenopausal women, Prophylaxis of postmenopausal osteoporosis Adult: 60 mg once daily.
|
|
Renal Impairment
Severe: Contraindicated.
|
|
Hepatic Impairment
Contraindicated.
|
|
Administration
May be taken with or without food.
|
|
Contraindications
Active or history of venous thromboembolic events including DVT, pulmonary embolism, retinal vein thrombosis. Unexplained uterine bleeding. Patients w/ signs/symptoms of endometrial cancer. Hepatic (including cholestasis) and severe renal impairment. Pregnancy, women who may become pregnant, and lactation.
|
|
Special Precautions
Women w/ risk factors for venous thromboembolism including CHF or active malignancy, risk factors for stroke (e.g. TIA, AF), history of oestrogen-induced hypertriglyceridaemia. Moderate renal impairment.
|
|
Adverse Reactions
Hot flushes, flu-like syndrome, leg cramps, peripheral oedema, sweating, arthralgia. Rarely, GI disturbances, rashes, thrombocytopenia, increased BP, headache including migraine, mild breast symptoms (e.g. tenderness, pain, enlargement).
Potentially Fatal: Venous thromboembolic events (including DVT, pulmonary embolism, retinal vein thrombosis), stroke. |
|
Monitoring Parameters
Monitor serum triglyceride concentration.
|
|
Drug Interactions
Reduced absorption and enterohepatic recycling w/ colestyramine. May reduce efficacy of warfarin.
|
|
Action
Description: Raloxifene is a selective oestrogen receptor modulator that has selective agonist or antagonist activities on oestrogen receptors. It acts as an agonist on bone by preventing bone loss and partially on cholesterol metabolism by decreasing total and LDL cholesterol levels, but not in the hypothalamus or in the breast or uterine tissues.
Onset: 8 wk. Pharmacokinetics: Absorption: Absorbed from the GI tract. Bioavailability: Approx 2%. Distribution: Extensively distributed in the body. Volume of distribution: 2,348 L/kg. Plasma protein binding: >95% (mainly to albumin and α1-acid glycoprotein). Metabolism: Undergoes extensive first-pass hepatic metabolism to glucuronide conjugates; undergoes enterohepatic recycling. Excretion: Mainly via faeces; urine (<0.2% as unchanged drug; <6% as glucuronide conjugates). Half-life: Approx 27 hr. |
|
Chemical Structure
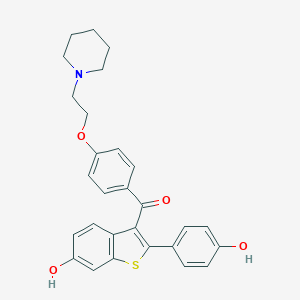 Source: National Center for Biotechnology Information. PubChem Database. Raloxifene, CID=5035, https://pubchem.ncbi.nlm.nih.gov/compound/Raloxifene (accessed on Jan. 22, 2020) |
|
Storage
Store between 20-25°C.
|
|
MIMS Class
|
|
ATC Classification
G03XC01 - raloxifene ; Belongs to the class of selective estrogen receptor modulators.
|
|
References
Anon. Raloxifene. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 03/02/2016. Buckingham R (ed). Raloxifene hydrochloride. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 03/02/2016. Joint Formulary Committee. Raloxifene hydrochloride. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 03/02/2016. McEvoy GK, Snow EK, Miller J et al (eds). Raloxifene hydrochloride. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 03/02/2016. Raloxifene Hydrochloride Tablet, Film Coated (Actavis Pharma, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 03/02/2016.
|