Urinary incontinence, urgency and frequency
Adult: Initially, 25 once daily; may increase to 50 mg once daily based on individual response and tolerance.
|
Indications and Dosage
Oral
Urinary incontinence, urgency and frequency Adult: Initially, 25 once daily; may increase to 50 mg once daily based on individual response and tolerance.
|
||||||
|
Renal Impairment
Haemodialysis patients: Contraindicated.
|
||||||
|
Hepatic Impairment
Moderate (Child-Pugh Class B): Max: 25 mg once daily. Severe (Child-Pugh Class C): Contraindicated.
|
||||||
|
Administration
XR tab: May be taken with or without food. Swallow whole, do not chew/crush/divide.
|
||||||
|
Contraindications
Severe uncontrolled HTN (systolic BP ≥180 mmHg or diastolic BP ≥110 mmHg). Severe hepatic impairment, ESRD, or haemodialysis patients. Lactation. Patients taking concomitant strong CYP3A inhibitors who have moderate to severe hepatic or severe renal impairment.
|
||||||
|
Special Precautions
Patient w/ clinically significant bladder outlet obstruction, history of QT-interval prolongation, stage 2 HTN. Hepatic and renal impairment. Pregnancy.
|
||||||
|
Adverse Reactions
Significant: HTN.
Nervous: Headache, dizziness. CV: Tachycardia, palpitations, AF. GI: Constipation, diarrhea, nausea, dry mouth, abdominal pain or distension, dyspepsia, gastritis. Resp: Nasopharyngitis, upper resp tract infection, sinusitis, rhinitis. Genitourinary: UTI, bladder pain, nephrolithiasis, vag infection, vulvovaginal pruritus. Musculoskeletal: Arthralgia. Ophthalmologic: Glaucoma. Dermatologic: Pruritus, rash, urticaria, purpura, leukocytoclastic vasculitis. Others: Fatigue. Potentially Fatal: Angioedema of the face, lips, tongue, and/or larynx. |
||||||
|
Monitoring Parameters
Monitor BP at baseline and regularly during therapy.
|
||||||
|
Overdosage
Symptoms: Palpitations, increased pulse rate and systolic BP. Management: Symptomatic and supportive treatment.
|
||||||
|
Drug Interactions
Increased exposure w/ strong CYP3A inhibitors (e.g. ketoconazole). May increase exposure to CYP2D6 substrates (e.g. desipramine, metoprolol), digoxin, and warfarin. Increased risk of urinary retention w/ antimuscarinic agents (e.g. solifenacin, darifenacin) due to additive pharmacologic effect.
|
||||||
|
Action
Description: Mirabegron relaxes detrusor smooth muscle in the bladder during the storage phase of micturition by selectively activating β3-adrenergic receptors, thereby increasing bladder capacity.
Onset: W/in 8 wk. Pharmacokinetics: Absorption: Bioavailability: 29-35%. Time to peak plasma concentration: Approx 3.5 hr. Distribution: Widely distributed in the body, including erythrocytes. Plasma protein binding: Approx 71%, mainly to albumin and α1-acid glycoprotein. Metabolism: Extensively metabolised via multiple pathways including dealkylation, oxidation, glucuronidation, and amide hydrolysis by multiple enzymes (e.g. butylcholinesterase, uridine diphospho-glucuronosyltransferase [UGT], CYP3A4, CYP2D6, and possibly by alcohol dehydrogenase) to form 2 major inactive metabolites. Excretion: Via urine (55% as radiolabeled drug and approx 25% as unchanged drug) and faeces (34% as radiolabeled drug). Terminal elimination half-life: Approx 50 hr. |
||||||
|
Chemical Structure
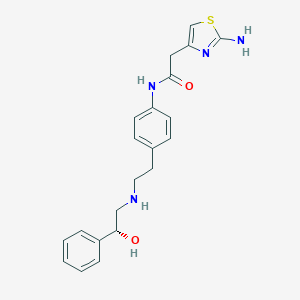 Source: National Center for Biotechnology Information. PubChem Database. Mirabegron, CID=9865528, https://pubchem.ncbi.nlm.nih.gov/compound/Mirabegron (accessed on Jan. 22, 2020) |
||||||
|
Storage
Store at 25°C.
Any unused portions should be disposed of in accordance w/ local requirements. |
||||||
|
MIMS Class
|
||||||
|
ATC Classification
G04BD12 - mirabegron ; Belongs to the class of urinary antispasmodics.
|
||||||
|
References
Anon. Mirabegron. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 02/05/2017 . Buckingham R (ed). Mirabegron. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 02/05/2017. Joint Formulary Committee. Mirabegron. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 02/05/2017 . McEvoy GK, Snow EK, Miller J et al (eds). Mirabegron. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 02/05/2017 . Myrbetriq Tablet, Film Coated, Extended Release (Astellas Pharma US, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 02/05/2017 .
|