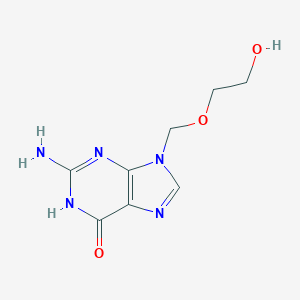
Recurrent herpes labialis
Adult: As mucoadhesive buccal tab: Apply 1 tab (50 mg) as a single dose to the upper gum region within 1 hour of onset of prodromal symptoms and before the appearance of any signs of herpes labialis lesions.
Intravenous
Mucocutaneous herpes simplex in immunocompromised patients
Adult: 5 mg/kg 8 hourly via slow infusion over 1 hour for 5-7 days.
Child: 3 months to 12 years 250 mg/m2 8 hourly via slow IV infusion over 1 hour for 5 days.
Child: 3 months to 12 years 250 mg/m2 8 hourly via slow IV infusion over 1 hour for 5 days.
Intravenous
First episodes of genital herpes
Adult: 5 mg/kg 8 hourly via slow IV infusion over 1 hour for 7 days.
Intravenous
Herpes simplex encephalitis
Adult: 10 mg/kg 8 hourly via slow IV infusion over 1 hour for 10 days.
Child: 3 months to 12 years 500 mg/m2 or 10 mg/kg via slow IV infusion over 1 hour.
Child: 3 months to 12 years 500 mg/m2 or 10 mg/kg via slow IV infusion over 1 hour.
Intravenous
Varicella-zoster virus infection
Adult: 5 mg/kg 8 hourly. Immunocompromised patient: 10 mg/kg 8 hourly. Doses to be given via slow IV infusion over 1 hour. Treatment duration: 5-7 days.
Child: 3 months to 12 years 250 mg/m2. Immunocompromised patient: 500 mg/m2. Doses to be given via slow IV infusion over 1 hour. Treatment duration: 5-7 days.
Child: 3 months to 12 years 250 mg/m2. Immunocompromised patient: 500 mg/m2. Doses to be given via slow IV infusion over 1 hour. Treatment duration: 5-7 days.
Intravenous
Neonatal herpes simplex virus infections
Child: 20 mg/kg IV 8 hourly for 14 days for mucocutaneous infections and 21 days for disseminated or CNS disease.
Ophthalmic
Herpes simplex keratitis
Adult: As 3% ointment: Apply 1 cm ribbon of ointment inside the lower conjunctival sac 5 times daily at approx 4-hour intervals. Continue treatment for at least 3 days after healing is complete.
Child: Same as adult dose.
Child: Same as adult dose.
Oral
Varicella-zoster virus infection
Adult: 800 mg 5 times daily at approx 4-hourly intervals, omit night time dose. Treatment duration: 7 days.
Child: ≥2 years 20 mg/kg/dose 4 times daily. Children >40 kg Same as adult dose. Alternative dose: <2 years 200 mg 4 times daily for 5 days; 2-5 years 400 mg 4 times daily for 5 days; 6-11 years 800 mg 4 times daily for 5 days; 12-17 years Same as adult dose. Max: 800 mg daily.
Child: ≥2 years 20 mg/kg/dose 4 times daily. Children >40 kg Same as adult dose. Alternative dose: <2 years 200 mg 4 times daily for 5 days; 2-5 years 400 mg 4 times daily for 5 days; 6-11 years 800 mg 4 times daily for 5 days; 12-17 years Same as adult dose. Max: 800 mg daily.
Oral
Primary herpes simplex infections
Adult: 200 mg 5 times daily at approx 4-hourly intervals, omit night time dose. Treatment duration: 5-10 days. Severely immunocompromised patients or patients with impaired absorption: Double the dose to 400 mg; consider IV dosing.
Child: <2 years Half the adult dose; ≥2 years Same as adult dose.
Child: <2 years Half the adult dose; ≥2 years Same as adult dose.
Oral
Suppression of recurrent herpes simplex
Adult: 400 mg bid at approx 12-hourly intervals. Alternatively, 200 mg 4 times daily at approx 6-hourly intervals. Treatment is interrupted every 6-12 months to re-assess recurrence frequency. Consider restarting after 2 or more recurrences. Intermittent therapy: 200 mg 5 times daily at approx 4-hourly intervals for 5 days, preferably initiated during the prodromal period.
Child: 12-17 years Same as adult dose.
Child: 12-17 years Same as adult dose.
Oral
Prophylaxis of herpes simplex in immunocompromised patients
Adult: 200 mg 4 times daily at approx 6-hourly intervals. Severely immunocompromised patients or patients with impaired absorption: Double the dose to 400 mg; consider IV dosing.
Child: <2 years Half the adult dose; ≥2 years Same as adult dose.
Child: <2 years Half the adult dose; ≥2 years Same as adult dose.
Oral
Herpes zoster (shingles)
Adult: 800 mg 5 times daily at approx 4-hourly intervals, omit night time dose. Treatment duration: 7-10 days.
Child: 20 mg/kg/dose 4 times daily. Children >40 kg Same as adult dose. <2 years 200 mg 4 times daily for 5 days; 2-5 years 400 mg 4 times daily for 5 days; 6-11 years 800 mg 4 times daily for 5 days; 12-17 years Same as adult dose.
Child: 20 mg/kg/dose 4 times daily. Children >40 kg Same as adult dose. <2 years 200 mg 4 times daily for 5 days; 2-5 years 400 mg 4 times daily for 5 days; 6-11 years 800 mg 4 times daily for 5 days; 12-17 years Same as adult dose.
Topical/Cutaneous
Genital herpes, Herpes labialis
Adult: As 5% cream: Apply 5 times daily at 4-hourly intervals for 4-10 days. As 5% ointment: Apply a sufficient amount to cover all lesions 6 times daily at 3-hourly intervals for 7 days. Start treatment preferably in the prodromal period.
Child: As 5% cream: Same as adult dose.
Child: As 5% cream: Same as adult dose.




 Sign Out
Sign Out