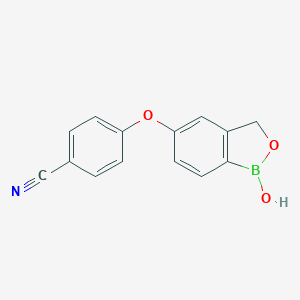
Atopic dermatitis
Adult: For the treatment of mild to moderate cases: As 2% ointment: Apply a thin layer to the affected area(s) bid.
Child: ≥3 months Same as adult dose.
Child: ≥3 months Same as adult dose.




 Sign Out
Sign Out