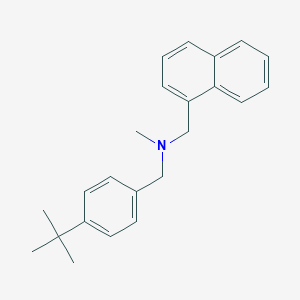
Skin fungal infections
Adult: As 1% cream: Tinea corporis and tinea cruris: Apply to affected and surrounding area once daily for 2 weeks. Tinea pedis: Apply to affected skin between and around the toes bid for 1 week or once daily for 4 weeks. Tinea versicolor: Apply to affected and surrounding area once daily for 1-2 weeks.
Child: ≥12 years Same as adult dose.
Child: ≥12 years Same as adult dose.




 Sign Out
Sign Out