Allergic rhinitis, Chronic idiopathic urticaria
Adult: 8 mg, as needed, up to tid. Max: 24 mg daily.
Child: ≥12 years Same as adult dose.
Child: ≥12 years Same as adult dose.
|
Indications and Dosage
Oral
Allergic rhinitis, Chronic idiopathic urticaria Adult: 8 mg, as needed, up to tid. Max: 24 mg daily.
Child: ≥12 years Same as adult dose. |
|
Renal Impairment
Severe: Contraindicated.
|
|
Administration
May be taken with or without food.
|
|
Contraindications
Severe renal impairment.
|
|
Special Precautions
Patient with porphyria. Mild to moderate renal impairment. Children and elderly. Pregnancy and lactation.
|
|
Adverse Reactions
Significant: CNS depression (e.g. drowsiness, dizziness).
Gastrointestinal disorders: Dry mouth. Nervous system disorders: Headache. Skin and subcutaneous tissue disorders: Rash. |
|
PO: Z (Adverse pregnancy outcomes (e.g. spontaneous abortions, intrauterine death) have been reported. Should not be used unless benefits outweigh risks.)
|
|
Patient Counseling Information
This drug may cause drowsiness and dizziness, if affected, do not drive or operate machinery.
|
|
Overdosage
Symptoms: Drowsiness, restlessness, hyperactivity, tachycardia. Management: Supportive treatment. May administer activated charcoal if needed.
|
|
Drug Interactions
Additive CNS depression with other CNS depressants, including sedatives and tranquilisers. Erythromycin and ketoconazole may increase the plasma concentration or adverse effects of acrivastine, respectively.
|
|
Food Interaction
May enhance the CNS depressant effect of alcohol.
|
|
Action
Description:
Mechanism of Action: Acrivastine, a non-sedating antihistamine, is structurally related to triprolidine. It is a competitive antagonist of H1-receptors but lacks significant anticholinergic effects. Additionally, it also has a low potential to penetrate the CNS. Onset: Inhibition of wheals/flares: 15 minutes. Symptom relief of allergic rhinitis: Within 1 hour. Duration: ≥8 hours (inhibition of wheals/flares). Pharmacokinetics: Absorption: Well absorbed from the gastrointestinal tract. Time to peak plasma concentration: 1.5 hours. Distribution: Plasma protein binding: 50%, mainly to albumin. Metabolism: Metabolised via reduction of acrylic acid side chain into propionic acid analogue. Excretion: Via urine (59% as unchanged drug, 15-17% as active metabolite); faeces. Elimination half-life: 1.5 hours. |
|
Chemical Structure
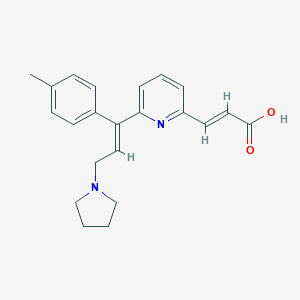 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 5284514, Acrivastine. https://pubchem.ncbi.nlm.nih.gov/compound/Acrivastine. Accessed Nov. 21, 2023. |
|
Storage
Store below 30°C.
|
|
MIMS Class
|
|
ATC Classification
R06AX18 - acrivastine ; Belongs to the class of other antihistamines for systemic use.
|
|
References
Anon. Acrivastine. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 20/06/2023. Benadryl Allergy Relief (McNeil Products Limited). MHRA. https://products.mhra.gov.uk. Accessed 20/06/2023. Brown & Burk Allergy Relief 8 mg Capsules, Hard (Brown & Burk UK Ltd.). MHRA. https://products.mhra.gov.uk. Accessed 20/06/2023. Buckingham R (ed). Acrivastine. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 20/06/2023. Joint Formulary Committee. Acrivastine. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 20/06/2023. Preston CL (ed). Acrivastine + Erythromycin. Stockley’s Drug Interactions [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 28/09/2023. Preston CL (ed). Acrivastine + Ketoconazole. Stockley’s Drug Interactions [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 28/09/2023.
|