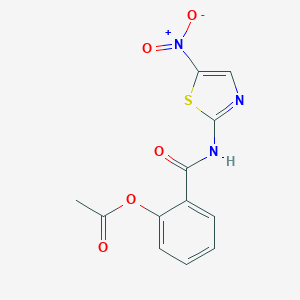
Cryptosporidiosis, Giardiasis
Adult: 500 mg 12 hourly for 3 days.
Child: 1-3 years 100 mg 12 hourly; 4-11 years 200 mg 12 hourly for 3 days. >12 years Same as adult dose. All doses to be taken for 3 days.
Child: 1-3 years 100 mg 12 hourly; 4-11 years 200 mg 12 hourly for 3 days. >12 years Same as adult dose. All doses to be taken for 3 days.




 Sign Out
Sign Out