Myotonic symptoms, Spastic paralysis
Adult: Usual dose: 50 mg tid; may be adjusted based on patient's age and symptoms. Treatment recommendations may vary among countries and individual products (refer to specific product guidelines).
|
Indications and Dosage
Oral
Myotonic symptoms, Spastic paralysis Adult: Usual dose: 50 mg tid; may be adjusted based on patient's age and symptoms. Treatment recommendations may vary among countries and individual products (refer to specific product guidelines).
|
|
Administration
Should be taken with food.
|
|
Contraindications
Myasthenia gravis. Lactation. Contraindications may vary among individual products (refer to specific product labelling).
|
|
Special Precautions
Hepatic impairment. Elderly. Pregnancy.
|
|
Adverse Reactions
Significant: Shock and anaphylactoid reactions (e.g. urticaria, pruritus, oedema of the face or other parts, dyspnoea), Stevens-Johnson syndrome, toxic epidermal necrolysis; weakness, dizziness, drowsiness.
Gastrointestinal disorders: Nausea, vomiting, diarrhoea, constipation, stomach discomfort, abdominal pain. General disorders and administration site conditions: Fatigue. Metabolism and nutrition disorders: Anorexia, increased thirst. Nervous system disorders: Headache, numbness of the extremities. Psychiatric disorders: Insomnia. Skin and subcutaneous tissue disorders: Rash. Vascular disorders: Hot flushes. |
|
Patient Counseling Information
This drug may cause sleepiness, weakness, or lightheadedness, if affected, do not drive or operate machinery.
|
|
Drug Interactions
May increase the risk of adverse effects of methocarbamol.
|
|
Action
Description:
Mechanism of Action: Eperisone is a centrally acting skeletal muscle relaxant that decreases muscle spindle sensitivity through the inhibition of spontaneous discharge of γ-motor neurons. It also exhibits vasodilatory action through antagonism of Ca influx. Pharmacokinetics: Absorption: Time to peak plasma concentration: 1.6-1.9 hours. Excretion: Elimination half-life: 1.6-1.8 hours. |
|
Chemical Structure
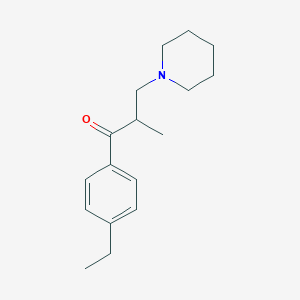 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 3236, Eperisone. https://pubchem.ncbi.nlm.nih.gov/compound/Eperisone. Accessed May 26, 2022. |
|
Storage
Store below 30°C. Protect from light and moisture.
|
|
MIMS Class
|
|
ATC Classification
M03BX09 - eperisone ; Belongs to the class of other centrally-acting muscle relaxants.
|
|
References
Anon. Eperisone. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 08/03/2022. Buckingham R (ed). Eperisone Hydrochloride. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 08/03/2022. Myelax 50 mg Tablet (The Cathay Drug Co., Inc.). MIMS Philippines. http://www.mims.com/philippines. Accessed 08/03/2022. Myonal 50 mg Tablet (HI-Eisai Pharmaceutical, Inc.). MIMS Philippines. http://www.mims.com/philippines. Accessed 08/03/2022. Myonal 50 mg Tablets (Esai [Malaysia] Sdn Bhd). National Pharmaceutical Regulatory Agency - Ministry of Health Malaysia. https://www.npra.gov.my. Accessed 08/03/2022. Perispa 50 mg Sugar Coated Tablet (Wert Philippines, Inc.). MIMS Philippines. http://www.mims.com/philippines. Accessed 28/04/2022.
|