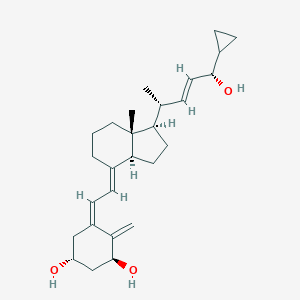
Mild to moderate plaque psoriasis
Adult: As 0.005% cream/oint: Apply a thin layer and rub in gently and completely to the affected area 1-2 times daily. Max: 100 g/wk; if used together w/ scalp soln, 5 mg of the drug per wk (e.g. cream/oint 60 g w/ scalp soln 40 mL or cream/oint 40 g w/ scalp soln 60 mL).
Child: As 0.005% oint: Apply bid. Max: 6-12 yr 50 g/wk; >12 yr 75 g/wk. Max duration: 8 wk.
Child: As 0.005% oint: Apply bid. Max: 6-12 yr 50 g/wk; >12 yr 75 g/wk. Max duration: 8 wk.
Topical/Cutaneous
Scalp psoriasis
Adult: As 0.005% soln: Apply and rub in gently and completely to the affected area bid. Avoid contact w/ the forehead or uninvolved scalp margin. Max: 60 mL/wk; if used together w/ cream/oint, 5 mg of the drug per wk (e.g. cream/oint 60 g w/ scalp soln 40 mL or cream/oint 40 g w/ scalp soln 60 mL).




 Sign Out
Sign Out