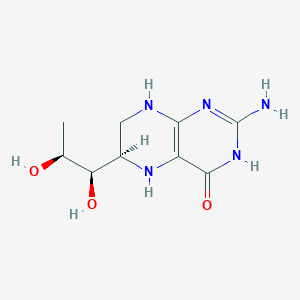
Hyperphenylalaninaemia due to phenylketonuria
Adult: As sapropterin dihydrochloride: Initially, 10 mg/kg once daily, preferably in the morning, adjusted according to desired phenylalanine levels. Usual maintenance dose: 5-20 mg/kg once daily.
Child: ≥4 years Same as adult dose.
Child: ≥4 years Same as adult dose.
Oral
Hyperphenylalaninaemia due to tetrahydrobiopterin (BH4) deficiency
Adult: As sapropterin dihydrochloride: Initially, 2-5 mg/kg once daily, preferably in the morning, adjusted according to desired phenylalanine levels. Max total dose: 20 mg/kg daily, may alternatively be given in 2-3 divided doses.
Child: ≥4 years Same as adult dose.
Child: ≥4 years Same as adult dose.




 Đăng xuất
Đăng xuất