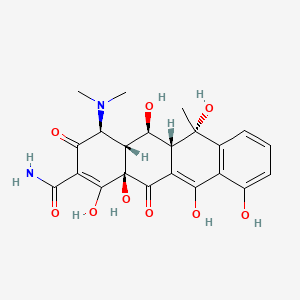
Brucellosis
Adult: In combination with streptomycin: 500 mg 4 times daily. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Rectal infection due to Chlamydia trachomatis, Uncomplicated genital infections due to Chlamydia trachomatis
Adult: 500 mg 4 times daily for 7 days. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Acne vulgaris
Adult: 250-500 mg in single or divided doses for at least 3 months. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Nongonococcal urethritis
Adult: For cases associated with Ureaplasma urealyticum: 500 mg 4 times daily for 7 days. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Epididymo-orchitis
Adult: For the treatment of acute cases associated with Chlamydia trachomatis or Neisseria gonorrhoeae: 500 mg 4 times daily for 10 days. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Rosacea
Adult: For the treatment of severe cases: 250-500 mg in single or divided doses for at least 3 months. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Primary syphilis, Secondary syphilis
Adult: As an alternative treatment: Early syphilis: 500 mg 4 times daily for 15 days. Latent syphilis of >1 year or of unknown duration, CV syphilis, or late benign syphilis (except neurosyphilis): 500 mg 4 times daily for 30 days. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Oral
Streptococcal infectious disease
Adult: Usual dosage range: 250-500 mg 6 hourly. Max treatment duration: 10 days. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.
Child: ≥12 years Same as adult dose. Dosage recommendations may vary among countries or individual products. Refer to specific product guidelines.




 Đăng xuất
Đăng xuất