Ulcerative colitis
Adult: Acute mild cases: Initially, 1 g daily in divided doses, gradually increased over 1 wk, according to patient’s response. Max: 3 g daily in divided doses (max/dose: 1 g). Remission maintenance: 500 mg bid.
|
Chỉ định và Liều dùng
Oral
Ulcerative colitis Adult: Acute mild cases: Initially, 1 g daily in divided doses, gradually increased over 1 wk, according to patient’s response. Max: 3 g daily in divided doses (max/dose: 1 g). Remission maintenance: 500 mg bid.
|
|
Cách dùng
Should be taken with food.
|
|
Chống chỉ định
Hypersensitivity to olsalazine or other salicylates.
|
|
Thận trọng
Patient w/ asthma, severe allergies. Renal and hepatic impairment. Pregnancy and lactation.
|
|
Tác dụng không mong muốn
Significant: Diarrhoea, ulcerative colitis symptoms exacerbation. Rarely, blood dyscrasias.
Nervous: Headache, dizziness, paraesthesia, depression, vertigo, fatigue, drowsiness, lethargy. CV: Tachycardia. GI: Nausea, vomiting, dyspepsia, abdominal cramps and pain, bloating, stomatitis, anorexia. Resp: Upper resp tract infection, dyspnoea. Hepatic: Increased hepatic enzyme. Haematologic: Thrombocytopenia. Musculoskeletal: Arthralgia, myalgia. Dermatologic: Rash, pruritus, alopecia, photosensitivity reaction, urticaria. Others: Pyrexia. Potentially Fatal: Liver necrosis, liver failure. |
|
Thông tin tư vấn bệnh nhân
This drug may cause dizziness and/or blurred vision, if affected, do not drive or operate machinery.
|
|
Chỉ số theo dõi
Monitor renal function (e.g. serum creatinine, BUN, urinalysis) prior and during treatment (every 3 mth for 1 yr, then every 6 mth for next 4 yr, then annually thereafter). Monitor CBC, LFT, stool frequency.
|
|
Quá liều
Symptoms: Nausea, vomiting, and diarrhoea. Management: Supportive treatment.
|
|
Tương tác
Increased risk of bleeding (e.g. haematoma) w/ LMWH or heparinoids. Increased prothrombin time w/ warfarin. Increased risk of myelosuppression w/ 6-mercaptopurine and thioguanine. Increased risk of developing Reye's syndrome w/ varicella vaccine, avoid admin w/in 6 wk of vaccination.
|
|
Tác dụng
Description:
Mechanism of Action: Olsalazine is a mesalazine [5-aminosalicylic acid (5-ASA)] derivative. The exact mechanism of action is still unknown but is thought to diminish inflammation by blocking cyclooxygenase and lipoxygenase pathways thus inhibiting the production of prostaglandin and leukotrienes in the colon; action appears to be topical rather than systemic. Pharmacokinetics: Absorption: Approx 2.4% is absorbed from the GI tract; 98-99% reaches the colon. Time to peak plasma concentration: Approx 1 hr. Distribution: Enters breast milk (5-ASA). Plasma protein binding: >99% (olsalazine-S), 74% (5-ASA), 81% (N-acetyl-5-ASA). Metabolism: Absorbed drug (approx 2% of the dose) is partially metabolised in the liver via sulfate conjugation to olsalazine-O-sulfate (olsalazine-S), while majority of the dose (98-99%) passes intact colon and cleaved by the intestinal flora to form 2 molecules of mesalazine (5-ASA), and metabolised further in the liver and colonic epithelium via N-acetylation to form N-acetyl-5-aminosalicylic acid (Ac-5-ASA). Excretion: Olsalazine-S: Mainly via urine. Mesalazine: Mainly via faeces (as Ac-5-ASA); urine (20-30%, mainly as Ac-5-ASA and 1-2% as unchanged drug). Elimination half-life: 7 days (olsalazine-S). |
|
Đặc tính
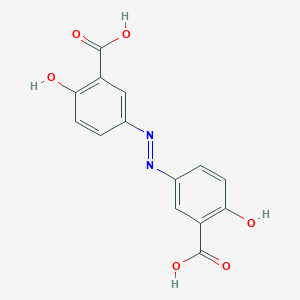 Source: National Center for Biotechnology Information. PubChem Database. Olsalazine, CID=22419, https://pubchem.ncbi.nlm.nih.gov/compound/Olsalazine (accessed on Jan. 22, 2020) |
|
Bảo quản
Store between 20-25°C.
|
|
Phân loại MIMS
|
|
Phân loại ATC
A07EC03 - olsalazine ; Belongs to the class of aminosalicylic acid and similar antiinflammatory. Used in the treatment of intestinal inflammation.
|
|
Tài liệu tham khảo
Anon. Olsalazine. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 23/08/2017. Buckingham R (ed). Olsalazine Sodium. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 23/08/2017. Dipentum Capsule, Gelatin Coated (Meda Pharmaceuticals). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 23/08/2017. Joint Formulary Committee. Olsalazine Sodium. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 23/08/2017. McEvoy GK, Snow EK, Miller J et al (eds). Olsalazine Sodium. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 23/08/2017.
|