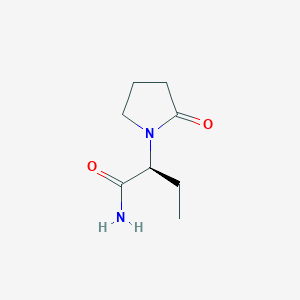
Partial seizures with or without secondary generalisation
Adult: As alternative when oral use is temporarily not feasible: Monotherapy in newly diagnosed patients: Initially, 250 mg bid, increased to the initial therapeutic dose of 500 mg bid after 2 weeks. Dose may be further increased by 250 mg bid every 2 weeks according to response. Max: 1,500 mg bid. Adjunctive therapy: Initially, 500 mg bid on the 1st day of treatment. Dose may be adjusted in 500 mg bid increases or decreases every 2-4 weeks according to response and tolerability. Max: 1,500 mg bid. All doses are given via infusion over 15 minutes. Max treatment duration: 4 days. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Monotherapy in newly diagnosed patients: ≥16 years Same as adult dose. Adjunctive therapy: 4-11 years and 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid. Use the lowest effective dose and most appropriate pharmaceutical form based on age, weight and dose; 12-17 years weighing ≥50 kg: Same as adult dose. All doses are given via infusion over 15 minutes. Max treatment duration: 4 days. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Monotherapy in newly diagnosed patients: ≥16 years Same as adult dose. Adjunctive therapy: 4-11 years and 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid. Use the lowest effective dose and most appropriate pharmaceutical form based on age, weight and dose; 12-17 years weighing ≥50 kg: Same as adult dose. All doses are given via infusion over 15 minutes. Max treatment duration: 4 days. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Intravenous
Myoclonic seizures
Adult: As alternative when oral use is temporarily not feasible: Adjunctive therapy in patients with juvenile myoclonic epilepsy: Initially, 500 mg bid on the 1st day of treatment. Dose may be adjusted in 500 mg bid increases or decreases every 2-4 weeks according to response and tolerability. Doses to be given via infusion over 15 minutes. Max: 1,500 mg bid. Max treatment duration: 4 days. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Adjunctive therapy in patients with juvenile myoclonic epilepsy: 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid; ≥50 kg: Same as adult dose. All doses are given via infusion over 15 minutes. Max treatment duration: 4 days. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Adjunctive therapy in patients with juvenile myoclonic epilepsy: 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid; ≥50 kg: Same as adult dose. All doses are given via infusion over 15 minutes. Max treatment duration: 4 days. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Intravenous
Primary generalised tonic-clonic seizures
Adult: As alternative when oral use is temporarily not feasible: Adjunctive therapy in patients with idiopathic generalised epilepsy: Initially, 500 mg bid on the 1st day of treatment. Dose may be adjusted in 500 mg bid increases or decreases every 2-4 weeks according to response and tolerability. Doses to be given via infusion over 15 minutes. Max: 1,500 mg bid. Max treatment duration: 4 days. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Adjunctive therapy in patients with idiopathic generalised epilepsy: 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid; ≥50 kg: Same as adult dose. All doses are given via infusion over 15 minutes. Max treatment duration: 4 days. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Adjunctive therapy in patients with idiopathic generalised epilepsy: 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid; ≥50 kg: Same as adult dose. All doses are given via infusion over 15 minutes. Max treatment duration: 4 days. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Oral
Primary generalised tonic-clonic seizures
Adult: Adjunctive therapy in patients with idiopathic generalised epilepsy: As immediate release: Initially, 500 mg bid on the 1st day of treatment. Dose may be adjusted in 500 mg bid increases or decreases every 2-4 weeks according to response and tolerability. Max: 1,500 mg bid. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Adjunctive therapy in patients with idiopathic generalised epilepsy: As immediate release: 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid; ≥50 kg: Same as adult dose. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Adjunctive therapy in patients with idiopathic generalised epilepsy: As immediate release: 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid; ≥50 kg: Same as adult dose. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Oral
Myoclonic seizures
Adult: Adjunctive therapy in patients with juvenile myoclonic epilepsy: As immediate release: Initially, 500 mg bid on the 1st day of treatment. Dose may be adjusted in 500 mg bid increases or decreases every 2-4 weeks according to response and tolerability. Max: 1,500 mg bid. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Adjunctive therapy in patients with juvenile myoclonic epilepsy: As immediate release: 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid; ≥50 kg: Same as adult dose. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: Adjunctive therapy in patients with juvenile myoclonic epilepsy: As immediate release: 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid; ≥50 kg: Same as adult dose. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Oral
Partial seizures with or without secondary generalisation
Adult: As immediate-release: Monotherapy in newly diagnosed patients: Initially, 250 mg bid, increased to the initial therapeutic dose of 500 mg bid after 2 weeks. Dose may be further increased by 250 mg bid every 2 weeks according to response. Max: 1,500 mg bid. Adjunctive therapy: Initially, 500 mg bid on the 1st day of treatment. Dose may be adjusted in 500 mg bid increases or decreases every 2-4 weeks according to response and tolerability. Max: 1,500 mg bid. As extended-release: Monotherapy or adjunctive therapy: Initially, 1,000 mg once daily, may be increased in increments of 1,000 mg every 2 weeks according to response and tolerability. Max: 3,000 mg once daily. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: As immediate-release: Monotherapy in newly diagnosed patients: ≥16 years Same as adult dose. Adjunctive therapy: 1 to <6 months Initially, 7 mg/kg bid; may be adjusted in increases or decreases of up to 7 mg/kg bid every 2 weeks according to response and tolerability. Max: 21 mg/kg bid; 6-23 months, 2-11 years, and 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid. Oral solution is preferred in infants <6 months and children <6 years, weighing <25 kg, those unable to swallow tab, or for doses <250 mg. Use the lowest effective dose and most appropriate pharmaceutical form based on age, weight and dose; 12-17 years weighing ≥50 kg: Same as adult dose. As extended-release: Monotherapy or adjunctive therapy: ≥12 years weighing ≥50 kg: Same as adult dose. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).
Child: As immediate-release: Monotherapy in newly diagnosed patients: ≥16 years Same as adult dose. Adjunctive therapy: 1 to <6 months Initially, 7 mg/kg bid; may be adjusted in increases or decreases of up to 7 mg/kg bid every 2 weeks according to response and tolerability. Max: 21 mg/kg bid; 6-23 months, 2-11 years, and 12-17 years weighing <50 kg: Initially, 10 mg/kg bid; may be adjusted in increases or decreases of up to 10 mg/kg bid every 2 weeks according to response and tolerability. Max: 30 mg/kg bid. Oral solution is preferred in infants <6 months and children <6 years, weighing <25 kg, those unable to swallow tab, or for doses <250 mg. Use the lowest effective dose and most appropriate pharmaceutical form based on age, weight and dose; 12-17 years weighing ≥50 kg: Same as adult dose. As extended-release: Monotherapy or adjunctive therapy: ≥12 years weighing ≥50 kg: Same as adult dose. Dosage recommendations may vary among individual products and between countries (refer to detailed product guideline).




 Đăng xuất
Đăng xuất