Schizophrenia
Adult: Initially, 1 mg bid. Titrate dose slowly in daily increments not exceeding 2 mg bid up to target dose range of 6-12 mg bid. Max: 24 mg daily. Re-titrate dose if therapy is interrupted for more than 3 days.
|
Chỉ định và Liều dùng
Oral
Schizophrenia Adult: Initially, 1 mg bid. Titrate dose slowly in daily increments not exceeding 2 mg bid up to target dose range of 6-12 mg bid. Max: 24 mg daily. Re-titrate dose if therapy is interrupted for more than 3 days.
|
|
Nhóm bệnh nhân đặc biệt
Patients taking strong CYP2D6 (e.g. fluoxetine, paroxetine) inhibitors or strong CYP3A4 (e.g. clarithromycin, ketoconazole) inhibitors: Reduce dose by 50%. Resume to previous dose upon discontinuation of CYP2D6 or CYP3A4 inhibitor treatment.
Pharmacogenomics: Iloperidone is metabolised by CYP2D6 and CYP3A4 isoenzymes to form its major metabolites, P88 and P95. The P95 metabolite represents 47.9% of iloperidone exposure in CYP2D6 extensive metabolisers and 25% in CYP2D6 poor metabolisers, while the P88 metabolite accounts 19.5% of total iloperidone plasma levels in CYP2D6 extensive metabolisers and 34% in CYP2D6 poor metabolisers. Individuals carrying reduced CYP2D6 activity, known as CYP2D6 poor metabolisers, may experience higher iloperidone exposure and an increased risk of QTc prolongation. However, researches that explore the association between CYP2D6 genotype and its clinical outcomes are limited. The prevalence of CYP2D6 poor metabolisers is approx 7-10% in Caucasians and approx 3-8% in African-Americans. The rest of population may be classified as intermediate, extensive or ultrarapid metabolisers. CYP2D6 poor metabolisers FDA recommends reducing dose by 50% as these individuals may have higher exposure to iloperidone compared with extensive metabolisers. Laboratory testing to identify CYP2D6 poor metabolisers is currently available. |
|
Suy gan
Moderate: Dose reduction may be necessary. Severe: Not recommended.
|
|
Chống chỉ định
Congenital long QT syndrome, history of cardiac arrhythmias, recent MI, uncompensated heart failure. Concomitant use with QTc-prolonging drugs.
|
|
Thận trọng
Patient with risk factors for blood dyscrasia (e.g. pre-existing low WBC, history of drug-induced leucopenia/neutropenia); pre-existing abnormal lipid profile; risk factors for aspiration pneumonia (e.g. Alzheimer’s disease); CV disease (e.g. heart failure, history of MI or ischaemia, conduction abnormalities), cerebrovascular disease, or predisposing conditions to hypotension (e.g. dehydration, hypovolaemia); conditions that increase risk of torsade de pointes or sudden death (e.g. bradycardia, hypokalaemia, hypomagnesaemia); history of seizures or conditions that may lower seizure threshold (e.g. Alzheimer’s dementia, head trauma, brain damage, alcoholism); Parkinson’s disease dementia or Lewy body dementia; diabetes mellitus, its risk factors (e.g. family history of diabetes, obesity), or other disorders of glucose regulation. Patients undergoing strenuous exercise or exposed to extreme heat. Avoid abrupt withdrawal. Renal and moderate to severe hepatic impairment. Elderly with dementia-related psychosis. Pregnancy and lactation. Concomitant use with strong CYP2D6 or CYP3A4 inhibitors. CYP2D6 poor metabolisers.
|
|
Tác dụng không mong muốn
Significant: Altered cardiac conduction and QTc interval prolongation, blood dyscrasias (e.g. leucopenia, neutropenia), CNS depression, dyslipidaemia, oesophageal dysmotility and aspiration; suicidal ideation, orthostatic hypotension associated with dizziness, tachycardia, and syncope; seizures, extrapyramidal symptoms, including pseudoparkinsonism, acute dystonic reactions, akathisia, and tardive dyskinesia; falls due to somnolence, and motor/sensory instability; hyperprolactinaemia, weight gain, impaired core body temperature regulation, withdrawal symptoms. Rarely, priapism.
Blood and lymphatic system disorders: Anaemia. Cardiac disorders: Palpitations. Eye disorders: Blurred vision, conjunctivitis. Gastrointestinal disorders: Nausea, dry mouth, diarrhoea, abdominal discomfort. General disorders and admin site conditions: Fatigue, lethargy, oedema. Investigations: Weight decreased. Metabolism and nutrition disorders: Increased appetite, dehydration, hypokalaemia. Musculoskeletal and connective tissue disorders: Arthralgia, myalgia, musculoskeletal stiffness, muscle spasms. Nervous system disorders: Tremor, paraesthesia. Psychiatric disorders: Restlessness, aggression, delusion, hostility, libido decreased. Renal and urinary disorders: Urinary incontinence. Reproductive system and breast disorders: Ejaculation disorder, erectile dysfunction, amenorrhoea, gynaecomastia, galactorrhoea. Respiratory, thoracic and mediastinal disorders: Nasal congestion, nasopharyngitis, upper respiratory tract infection, dyspnoea. Skin and subcutaneous tissue disorders: Rash, pruritus. Vascular disorders: Hypotension. Potentially Fatal: Arrhythmias, agranulocytosis, cerebrovascular effects (e.g. transient ischaemic attack, stroke), neuroleptic malignant syndrome (NMS), agranulocytosis, aspiration pneumonia, hyperglycaemia associated with ketoacidosis or hyperosmolar coma; hypersensitivity reactions (e.g. anaphylaxis, angioedema). |
|
Thông tin tư vấn bệnh nhân
This drug may cause somnolence, and impaired judgment, thinking or motor skills, if affected, do not drive or operate machinery.
|
|
Chỉ số theo dõi
Monitor blood pressure, fasting plasma glucose level, or HbA1c, and fasting lipid panel at baseline, repeated 3 months after initiation, then yearly thereafter; weight, height, BMI, and waist circumference at baseline, repeated at 4, 8, and 12 weeks after starting or changing treatment; serum electrolytes (e.g. K, Mg) at baseline, annually and as clinically indicated (periodically in at risk patients); LFTs annually and as clinically indicated; CBC, ECG and vital signs as clinically indicated. Perform ocular exam at the start of treatment then yearly. Assess mental status for depression, suicidal ideation; changes in menstruation, libido, development of galactorrhoea, erectile and ejaculatory function, tardive dyskinesia, abnormal involuntary movements, and parkinsonian signs.
|
|
Quá liều
Symptoms: Exaggeration of known pharmacologic effects, such as drowsiness, sedation, tachycardia, hypotension, extrapyramidal symptoms, and QTc interval prolongation. Management: Symptomatic and supportive treatment. Establish and maintain airway; ensure adequate oxygenation and ventilation. Consider gastric lavage after intubation for unconscious patients and administration of activated charcoal with laxative. Initiate CV monitoring immediately to detect possible arrhythmias, then administer appropriate antiarrhythmic treatment if needed. Administer IV fluids and sympathomimetic agents for hypotension and circulatory collapse; anticholinergic agents for severe extrapyramidal symptoms.
|
|
Tương tác
Increased blood levels with strong CYP3A4 (e.g. ketoconazole, clarithromycin) and CYP2D6 (e.g. fluoxetine, paroxetine) inhibitors. May increase total exposures of dextromethorphan, midazolam. May enhance effects of certain antihypertensive agents. Impaired core body temperature regulation with anticholinergic agents.
Potentially Fatal: Increased risk of QT prolongation with drugs known to prolong QTc, such as Class 1A (e.g. quinidine, procainamide) or Class III (e.g. amiodarone, sotalol) antiarrhythmics, certain antipsychotic agents (e.g. chlorpromazine, thioridazine), certain antibiotics (e.g. moxifloxacin, gatifloxacin), methadone, pentamidine, levomethadyl acetate. |
|
Tương tác với thức ăn
May enhance CNS depressant effects of alcohol.
|
|
Tác dụng
Description:
Mechanism of Action: Iloperidone is a piperidinyl benzisoxazole atypical antipsychotic with antagonistic activity at dopamine D2 and serotonin (5-HT2), D3, and α1-adrenergic receptors. The combined antagonism is thought to improve the negative symptoms of psychoses and lower the incidence of extrapyramidal adverse effects. Pharmacokinetics: Absorption: Well absorbed from the gastrointestinal tract. Time to peak plasma concentration: 2-4 hours. Distribution: Volume of distribution: 1,340-2,800 L. Bioavailability: 96%. Plasma protein binding: Approx 97% (iloperidone); approx 92% (P88 and P95). Metabolism: Metabolised in the liver primarily via carbonyl reduction, hydroxylation by CYP2D6 isoenzyme, and via O-demethylation by CYP3A4 isoenzyme to form its active metabolites, P88 and P95. Excretion: Mainly via urine (58% extensive metabolisers, 45% poor metabolisers, as P88 and P95; <1% as unchanged drug); faeces (20% extensive metabolisers, 22% poor metabolisers, as P88 and P95; <1% as unchanged drug). Elimination half-life: Extensive metabolisers: 18 hours (iloperidone); 26 hours (P88); 23 hours (P95). Poor metabolisers: 33 hours (iloperidone); 37 hours (P88); 31 hours (P95). |
|
Đặc tính
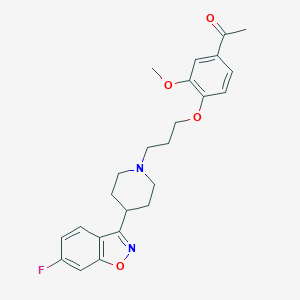 Source: National Center for Biotechnology Information. PubChem Database. Iloperidone, CID=71360, https://pubchem.ncbi.nlm.nih.gov/compound/Iloperidone (accessed on Mar. 24, 2020) |
|
Bảo quản
Store at 25°C. Protect from light and moisture.
|
|
Phân loại MIMS
|
|
Phân loại ATC
N05AX14 - iloperidone ; Belongs to the class of other antipsychotics.
|
|
Tài liệu tham khảo
Annotation of FDA Label for Iloperidone and CYP2D6. Pharmacogenomics Knowledgebase (PharmGKB). https://www.pharmgkb.org/. Accessed 13/12/2019. Anon. CYP2D6 - Iloperidone (Pharmacogenomics). Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 13/12/2019. Anon. Iloperidone. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 13/12/2019. Anon. Iloperidone. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 13/12/2019. Buckingham R (ed). Iloperidone. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 13/12/2019. Fanapt Tablet (Vanda Pharmaceuticals Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 13/12/2019. Fanapt Tablets (Vanda Pharmaceuticals Inc.). U.S. FDA. https://www.fda.gov/. Accessed 13/12/2019.
|