Acute bacterial exacerbation of chronic bronchitis
Adult: 320 mg once daily for 5 days.
Oral
Community-acquired pneumonia
Adult: Mild to moderate: 320 mg once daily for 7 days.
|
Chỉ định và Liều dùng
Oral
Acute bacterial exacerbation of chronic bronchitis Adult: 320 mg once daily for 5 days. Oral Community-acquired pneumonia Adult: Mild to moderate: 320 mg once daily for 7 days.
|
||||
|
Suy thận
|
||||
|
Cách dùng
May be taken with or without food.
|
||||
|
Chống chỉ định
Known hypersensitivity to gemifloxacin, other quinolones.
|
||||
|
Thận trọng
Patient w/ known or suspected CNS disorders (e.g. seizure disorders) or other risk factors predisposing to seizures, myasthenia gravis, previous tendon disorders (e.g. rheumatoid arthritis), history of QT interval prolongation, significant bradycardia or acute myocardial ischaemia, uncorrected electrolyte disorders. Kidney, heart or lung transplant recipients. Renal impairment. Pregnancy and lactation.
|
||||
|
Tác dụng không mong muốn
Significant: Increased risk of mental health adverse effects (e.g. disturbance in attention, disorientation, agitation, nervousness, memory impairment, delirium); blood glucose disturbance and risk of coma with hypoglycaemia, prolonged QT interval, photosensitivity/phototoxicity, tendinitis, tendon rupture, peripheral neuropathy (may be irreversible).
Blood and lymphatic system disorders: Leucopenia thrombocythemia. Cardiac disorders: Supraventricular tachycardia. Gastrointestinal disorders: Diarrhoea, nausea, vomiting, abdominal pain, constipation, flatulence, dyspepsia, dry mouth, gastritis. General disorders and administration site conditions: Fatigue. Infections and infestations: Fungal infection, genital moniliasis and pruritus, vaginitis. Metabolism and nutrition disorders: Anorexia, increased alkaline phosphatase, ALT, AST and creatine phosphokinase (CK). Nervous system disorders: Seizures, increased intracranial pressure, nightmares, dizziness, tremors. Psychiatric disorders: Insomnia, anxiety, depression, toxic psychosis, paranoia, confusion. Skin and subcutaneous tissue disorders: Rash, urticaria, dermatitis. Vascular disorders: Syncope. Potentially Life-threatening: Hypersensitivity reactions (e.g. anaphylaxis). |
||||
|
Thông tin tư vấn bệnh nhân
May impair ability to perform activities that require mental alertness or coordination (e.g. driving, operating machinery). Rest and refrain from doing strenuous physical activity as it may increase risk of tendon rupture. Avoid excessive exposure to sunlight or artificial UV light (e.g. tanning beds) and use protective measures (e.g. sunscreen, wear loose-fitting clothes) if staying outdoors is necessary during therapy.
|
||||
|
Chỉ số theo dõi
Monitor WBC, signs and symptoms of infection and renal function.
|
||||
|
Tương tác
Additive effect on QT interval prolongation w/ class IA (e.g. quinidine) or class III (e.g. amiodarone) antiarrhythmics and other drugs that prolong QT interval (e.g. erythromycin, TCAs, antipsychotic agents). Renal clearance reduced w/ probenecid. Decreased absorption w/ Al or Mg-containing antacids, buffered didanosine, sucralfate or dietary supplements containing metal cations (e.g. Zn, Mg, Fe). Increased risk of severe tendon disorders esp in elderly (>60 yr) w/ corticosteroids. May increase prothrombin time, INR and/or bleeding w/ warfarin.
|
||||
|
Tác dụng
Description:
Mechanism of Action: Gemifloxacin, a synthetic fluoroquinolone anti-infective agent, acts by inhibiting DNA synthesis in susceptible organisms via inhibition of both DNA gyrase and topoisomerase IV which are essential for bacterial growth. Pharmacokinetics: Absorption: Rapidly absorbed from the GI tract. Absolute bioavailability: Approx 71%. Time to peak plasma concentration: 0.5-2 hr. Distribution: Widely distributed into body tissues including bronchial mucosa and lungs. Volume of distribution: 4.2 L/kg. Plasma protein binding: Approx 55-73%. Metabolism: Undergoes limited hepatic metabolism. Excretion: Via faeces (61%) and urine (36%) as unchanged drug and metabolites. Elimination half-life: Approx 7 hr. |
||||
|
Đặc tính
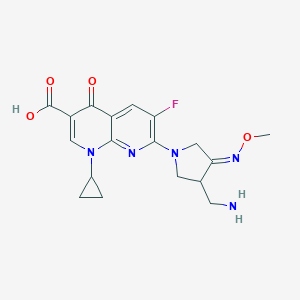 Source: National Center for Biotechnology Information. PubChem Database. Gemifloxacin, CID=9571107, https://pubchem.ncbi.nlm.nih.gov/compound/Gemifloxacin (accessed on Jan. 22, 2020) |
||||
|
Bảo quản
Store between 15-30°C. Protect from light.
|
||||
|
Phân loại MIMS
|
||||
|
Tài liệu tham khảo
Anon. Gemifloxacin. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 13/05/2014. Anon. Gemifloxacin. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 10/12/2018. Anon. Gemifloxacin. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 13/05/2014. Buckingham R (ed). Gemifloxacin Mesilate. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 13/05/2014. Factive (Gemifloxacin Mesylate) Tablets. U.S. FDA. https://www.fda.gov/. Accessed 13/05/2014. Factive (Merus Lab International Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 17/10/2018. Factive (Oscient Pharmaceuticals). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 13/05/2014. FDA Updates Warnings for Fluoroquinolone Antibiotics on Risks of Mental Health and Low Blood Sugar Adverse Reactions. U.S. FDA. https://www.fda.gov/. Accessed 12/10/2018. Wickersham RM. Gemifloxacin Mesylate. Facts and Comparisons [online]. St. Louis, MO. Wolters Kluwer Clinical Drug Information, Inc. https://www.wolterskluwercdi.com/facts-comparisons-online/. Accessed 13/05/2014.
|