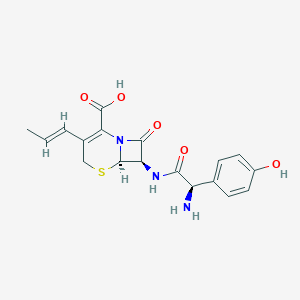
Pharyngitis, Tonsillitis
Adult: 500 mg once daily for 10 days.
Child: 2-12 years 7.5 mg/kg 12 hourly for 10 days. Max: 500 mg/dose; ≥13 years Same as adult dose.
Child: 2-12 years 7.5 mg/kg 12 hourly for 10 days. Max: 500 mg/dose; ≥13 years Same as adult dose.
Oral
Acute bacterial exacerbation of chronic bronchitis
Adult: 500 mg 12 hourly for 10 days.
Oral
Uncomplicated skin and skin structure infections
Adult: 250 or 500 mg 12 hourly, or 500 mg once daily. Treatment duration: 10 days.
Child: 2-12 years 20 mg/kg once daily. Max: 500 mg/dose; ≥13 years Same as adult dose.
Child: 2-12 years 20 mg/kg once daily. Max: 500 mg/dose; ≥13 years Same as adult dose.




 Đăng xuất
Đăng xuất