Acne vulgaris
Adult: As 1% cream/gel: Apply to affected area bid. Discontinue treatment if desired effect is not achieved after 4 weeks.
|
Indications and Dosage
Topical/Cutaneous
Acne vulgaris Adult: As 1% cream/gel: Apply to affected area bid. Discontinue treatment if desired effect is not achieved after 4 weeks.
|
|
Contraindications
Hypersensitivity to nadifloxacin or other fluoroquinolones.
|
|
Adverse Reactions
Skin and subcutaneous tissue disorders: Itching and erythema at application sites, pruritus, redness, flushing, feeling of facial warmth, papules, contact dermatitis, dry skin.
|
|
Action
Description: Nadifloxacin, a synthetic fluoroquinolone anti-infective agent, acts by inhibiting the bacterial DNA gyrase which is involved in DNA synthesis and replication, thus inhibiting bacterial multiplication
Pharmacokinetics: Absorption: Time to peak plasma concentration: 8 hours (post-final dosing). Excretion: Via urine (approx 0.09%). Elimination half-life: 19.4 hours. |
|
Chemical Structure
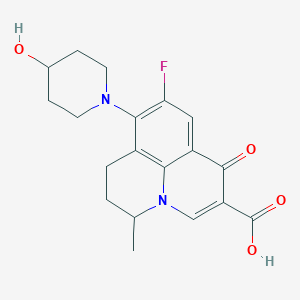 Source: National Center for Biotechnology Information. PubChem Database. Nadifloxacin, CID=4410, https://pubchem.ncbi.nlm.nih.gov/compound/Nadifloxacin (accessed on Mar. 25, 2020) |
|
Storage
Store below 30°C.
|
|
MIMS Class
|
|
ATC Classification
D10AF05 - nadifloxacin ; Belongs to the class of topical antiinfective preparations used in the treatment of acne.
|
|
References
Acuatim (Otsuka). MIMS Indonesia. http://mims.com/indonesia. Accessed 17/01/2020. Anon. Nadifloxacin. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 17/01/2020. Buckingham R (ed). Nadifloxacin. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 17/01/2020. Nadibact (Cipla - Xterna). MIMS Philippines. http://mims.com/philippines. Accessed 17/01/2020. Quinolones. Gold Standard Drug Database in ClinicalKey [online]. Elsevier Inc. https://www.clinicalkey.com/. Accessed 17/01/2020.
|