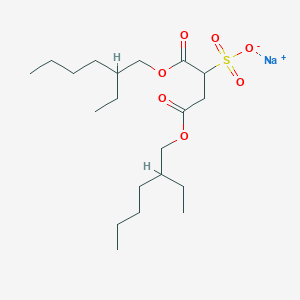
Chronic constipation
Adult: Prevention and treatment: As cap or 50 mg/5 mL oral solution: Up to Max of 500 mg daily in divided doses. Treatment must be started with large doses (e.g. 100 mg tid) according to response, then decreased as the patient condition improves. Dosage recommendations may vary among individual products or between countries (refer to detailed product guideline).
Child: As 12.5 mg/5 mL oral solution: Infants >6 months 12.5 mg tid; Children 12.5-25 mg tid. Dilute dose in a glass of flavoured drink or milk then take within 30 minutes of preparation. As cap: ≥12 years Same as adult dose. Dosage recommendations may vary among individual products or between countries (refer to detailed product guideline).
Child: As 12.5 mg/5 mL oral solution: Infants >6 months 12.5 mg tid; Children 12.5-25 mg tid. Dilute dose in a glass of flavoured drink or milk then take within 30 minutes of preparation. As cap: ≥12 years Same as adult dose. Dosage recommendations may vary among individual products or between countries (refer to detailed product guideline).
Oral
Adjunct in abdominal radiological procedures
Adult: As 50 mg/5 mL oral solution: 400 mg to be given with barium meal.
Child: As 12.5 mg/5 mL oral solution: 75 mg to be given with barium meal.
Child: As 12.5 mg/5 mL oral solution: 75 mg to be given with barium meal.
Otic/Aural
Softening of ear wax
Adult: As 0.5% or 5% ear drops: Apply a sufficient amount to fill the affected ear(s) once at night for up to 2 consecutive nights, before syringing with warm water if needed.
Child: As 0.5% or 5% ear drops: Same as adult dose.
Child: As 0.5% or 5% ear drops: Same as adult dose.
Rectal
Constipation
Adult: As enema: 120 mg as a single dose; another dose may be used on the same or the next day if necessary. Alternatively, 283 mg (1 enema) 1-3 times daily may be used. Dosage recommendations may vary among individual products or between countries (refer to detailed product guideline).
Child: ≥12 years As enema: Same as adult dose.
Child: ≥12 years As enema: Same as adult dose.




 Sign Out
Sign Out