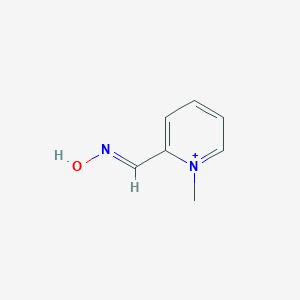
Organophosphorus poisoning
Adult: Mild: 600 mg, repeat 1-2 times every 15 minutes as needed, up to Max: 1,800 mg. Severe: 3 inj of 600 mg in rapid succession to a total dose of 1,800 mg. Persistent: May repeat the entire series (1,800 mg) starting approx 1 hour after administration of the last inj.
Child: <40 kg: Mild: 15 mg/kg/dose, repeated every 15 minutes as needed. Max: 45 mg/kg. Severe: 15 mg/kg/dose, repeated 2 times in rapid succession to a total dose of 45 mg/kg. Persistent: May repeat the entire series (45 mg/kg given in 3 divided doses) starting approx 1 hour after administration of the last inj. ≥40 kg: Same as adult dose.
Child: <40 kg: Mild: 15 mg/kg/dose, repeated every 15 minutes as needed. Max: 45 mg/kg. Severe: 15 mg/kg/dose, repeated 2 times in rapid succession to a total dose of 45 mg/kg. Persistent: May repeat the entire series (45 mg/kg given in 3 divided doses) starting approx 1 hour after administration of the last inj. ≥40 kg: Same as adult dose.
Intravenous
Organophosphorus poisoning
Adult: As adjunct to atropine: Loading dose: 1,000-2,000 mg via infusion over 15-30 minutes or slow inj over at least 5 minutes, may repeat dose after 1 hour, then 10-12 hourly as needed. For intermittent infusion dosing, repeat dose 4-6 hourly as needed. Max intermittent infusion rate: 200 mg/minute. Administer as soon as the effects of atropine are observed. Maintain atropinisation for at least 48 hours.
Child: ≤16 years As adjunct to atropine: Loading dose: 20-50 mg/kg (Max: 2,000 mg/dose) via inj over 15-30 minutes, followed by 10-20 mg/kg/hour as continuous infusion. Alternatively, a repeat bolus of 20-50 mg/kg after 1 hour and repeated 10-12 hourly as needed. >16 years Same as adult dose.
Child: ≤16 years As adjunct to atropine: Loading dose: 20-50 mg/kg (Max: 2,000 mg/dose) via inj over 15-30 minutes, followed by 10-20 mg/kg/hour as continuous infusion. Alternatively, a repeat bolus of 20-50 mg/kg after 1 hour and repeated 10-12 hourly as needed. >16 years Same as adult dose.
Intravenous
Anticholinesterase overdose
Adult: Initially, 1,000-2,000 mg, followed by 500-1,000 mg/hour via infusion. Alternatively, repeat initial dose after 1 hour then 3-8 hourly as needed.




 Sign Out
Sign Out