Ocular infections
Adult: As 1% ophthalmic ointment: Apply sparingly into the affected eye(s) 3-4 times daily. Dosage recommendations may vary among individual products or between countries (refer to detailed product guidelines).
|
Indications and Dosage
Ophthalmic
Ocular infections Adult: As 1% ophthalmic ointment: Apply sparingly into the affected eye(s) 3-4 times daily. Dosage recommendations may vary among individual products or between countries (refer to detailed product guidelines).
|
|
Contraindications
Hypersensitivity.
|
|
Adverse Reactions
General disorders and administration site conditions: Local irritation at the site of application.
Immune system disorders: Hypersensitivity reaction. Skin and subcutaneous tissue disorders: Photosensitivity, skin rash. |
|
Action
Description:
Mechanism of Action: Chlortetracycline is a tetracycline derivative that prevents protein synthesis by binding with the 30S and possibly 50S ribosomal subunit(s) of susceptible bacteria. It has a broad-spectrum antimicrobial activity against Gram-positive and Gram-negative organisms. |
|
Chemical Structure
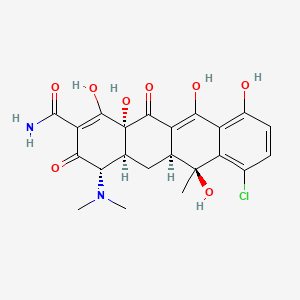 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 54675777, Chlortetracycline. https://pubchem.ncbi.nlm.nih.gov/compound/Chlortetracycline. Accessed May 30, 2023. |
|
Storage
Store below 30°C. Protect from light.
|
|
MIMS Class
|
|
ATC Classification
S01AA02 - chlortetracycline ; Belongs to the class of antibiotics. Used in the treatment of eye infections.
|
|
References
Anon. Chlortetracycline. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 04/05/2023. Buckingham R (ed). Chlortetracycline. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 04/05/2023. Chlortralim (Atlantic Laboratories Corp., Ltd). MIMS Thailand. http://www.mims.com/thailand. Accessed 19/05/2023. Chlortralim Ophthalmic Ointment (Atlantic Laboratories [M] Sdn Bhd). National Pharmaceutical Regulatory Agency - Ministry of Health Malaysia. https://www.npra.gov.my. Accessed 04/05/2023.
|