Chronic asthma
Adult: 20 mg bid.
Child: 5-11 years old 10 mg bid. ≥12 years old Same as adult dose.
Child: 5-11 years old 10 mg bid. ≥12 years old Same as adult dose.
|
Indications and Dosage
Oral
Chronic asthma Adult: 20 mg bid.
Child: 5-11 years old 10 mg bid. ≥12 years old Same as adult dose. |
|
Hepatic Impairment
Contraindicated.
|
|
Administration
Should be taken on an empty stomach. Take at least 1 hr before or 2 hr after meals.
|
|
Contraindications
Hepatic impairment including hepatic cirrhosis.
|
|
Special Precautions
Not indicated for use in the reversal of bronchospasm in acute asthma attacks. Avoid abruptly substitution to oral or inhaled corticosteroids. Renal impairment. Pregnancy and lactation.
|
|
Adverse Reactions
Systemic eosinophilia, eosinophilic pneumonia or w/ clinical features of systemic vasculitis consistent w/ Churg-Strauss syndrome; neuropsychiatric adverse events (e.g. insomnia, depression); headache, resp tract infection, GI disturbances, arthralgia, myalgia, fever, malaise, insomnia, dizziness, elevated liver enzyme values, hypersensitivity reactions (e.g. rashes, pruritus, urticaria, angioedema), agranulocytosis, bleeding, bruising, oedema.
Potentially Fatal: Severe hepatotoxicity. |
|
Monitoring Parameters
Monitor for improvements in air flow; monitor closely for sign/symptoms of hepatic injury; periodic monitoring of LFTs.
|
|
Overdosage
Symptoms: Rash and stomach upset. Management: Supportive and symptomatic treatment. Empty the stomach by inducing emesis or by gastric lavage. May administer activated charcoal to prevent absorption of unrecovered drug.
|
|
Drug Interactions
May enhance anticoagulant activity of warfarin resulting to increased prothrombin time. Decreased plasma levels w/ theophylline, terfenadine and erythromycin. Increased plasma levels w/ fluconazole and high dose aspirin.
|
|
Food Interaction
Food reduces the rate and extent of absorption.
|
|
Action
Description: Zafirlukast is a selective and competitive leukotriene-receptor antagonist (LTRA) of leukotriene D4 and E4 (LTD4 and LTE4), components of slow-reacting substance of anaphylaxis (SRSA). Cysteinyl leukotriene production and receptor occupation have been correlated w/ the pathophysiology of asthma, including airway oedema, smooth muscle constriction, and altered cellular activity associated w/ the inflammatory process, which contribute to the signs and symptoms of asthma.
Pharmacokinetics: Absorption: Rapidly absorbed from the GI tract. Food reduces the rate and extent of absorption. Time to peak plasma concentration: Approx 3 hr. Distribution: Volume of distribution: Approx 70 L. Plasma protein binding: Approx 99% (primarily to albumin). Metabolism: Extensively metabolised in the liver, mainly by CYP2C9 isoenzyme. Excretion: Mainly via faeces (approx 90%) as unchanged drug and metabolites and urine (approx 10%) as metabolites. Terminal elimination half-life: Approx 10 hr. |
|
Chemical Structure
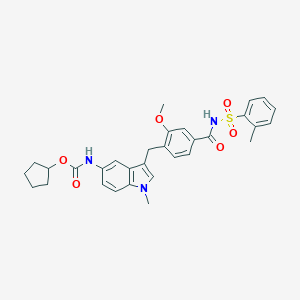 Source: National Center for Biotechnology Information. PubChem Database. Zafirlukast, CID=5717, https://pubchem.ncbi.nlm.nih.gov/compound/Zafirlukast (accessed on Jan. 24, 2020) |
|
Storage
Store between 20-25°C. Protect from light and moisture.
|
|
MIMS Class
|
|
References
Accolate Tablet. U.S. FDA. https://www.fda.gov/. Accessed 30/09/2014. Anon. Zafirlukast. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 01/10/2014. Buckingham R (ed). Zafirlukast. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 30/09/2014. McEvoy GK, Snow EK, Miller J et al (eds). Zafirlukast. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 30/09/2014. Zafirlukast Tablet, Film-Coated (AstraZeneca Pharmaceuticals LP). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 30/09/2014.
|