Locally advanced basal cell carcinoma, Metastatic basal cell carcinoma
Adult: 150 mg once daily until disease progression or unacceptable toxicity. If a dose is missed, skip the missed dose and wait until the next scheduled dose.
|
Indications and Dosage
Oral
Locally advanced basal cell carcinoma, Metastatic basal cell carcinoma Adult: 150 mg once daily until disease progression or unacceptable toxicity. If a dose is missed, skip the missed dose and wait until the next scheduled dose.
|
|
Administration
May be taken with or without food.
|
|
Contraindications
Pregnancy and lactation.
|
|
Special Precautions
Severe renal impairment.
|
|
Adverse Reactions
Significant: Amenorrhea, nausea, vomiting, diarrhoea, constipation, abdominal pain, decreased appetite, premature fusion of epiphyses in paediatric patients.
Gastrointestinal disorders: Dysgeusia, ageusia, hypogeusia, dyspepsia. General disorders and administration site conditions: Fatigue, pain asthenia. Investigations: Weight loss, increased hepatic enzymes, increased blood creatinine phosphokinase. Metabolism and nutrition disorders: Dehydration. Musculoskeletal and connective tissue disorders: Muscle spasm, arthralgia, pain in extremity, back pain, chest pain, myalgia, flank pain. Skin and subcutaneous tissue disorders: Alopecia, pruritus, rash, madarosis, abnormal hair growth. |
|
Patient Counseling Information
Avoid blood or sperm donation during therapy and for at least 24 months or 3 months, respectively, following drug discontinuation.
|
|
Monitoring Parameters
Perform pregnancy test within 1 week prior to initiation of therapy and monthly thereafter.
|
|
Drug Interactions
Decreased plasma concentration and decreased efficacy with CYP enzyme inducers (e.g. rifampicin, carbamazepine, phenytoin). May increase the exposure of BCRP substrates (e.g. sulfasalazine, rosuvastatin, topotecan) and OATP1B1 substrates (e.g. repaglinide, bosentan, valsartan, statins).
|
|
Food Interaction
Decreased plasma concentration with St John’s wort, avoid concomitant use.
|
|
Action
Description: Vismodegib inhibits hedgehog pathway signalling by binding to and inhibiting Smoothened homologue (SMO) transmembrane protein, thereby blocking the activation and nuclear localisation of Glioma-Associated Oncogene (GLI) transcription factors, and the induction of hedgehog target genes which are involved in the proliferation, survival and differentiation of basal cancer cells.
Pharmacokinetics: Absorption: Absorbed from the gastrointestinal tract. Absolute bioavailability: Approx 32%. Time to peak plasma concentration: Approx 2-4 days. Distribution: Volume of distribution: 16.4-26.6 L. Plasma protein binding: >99%, mainly to albumin and alpha1-acid glycoprotein. Metabolism: Metabolised in the liver via oxidation, glucuronidation and pyridine ring cleavage. Excretion: Mainly via faeces (approx 82%); urine (approx 4.4%). Elimination half-life: Approx 12 days (single dose); approx 4 days (continuous daily dosing). |
|
Chemical Structure
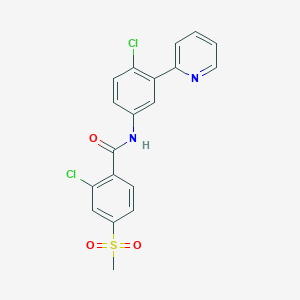 Source: National Center for Biotechnology Information. PubChem Database. Vismodegib, CID=24776445, https://pubchem.ncbi.nlm.nih.gov/compound/Vismodegib (accessed on Jan. 24, 2020) |
|
Storage
Store between 20-25°C. Protect from moisture.
This is a cytotoxic drug. Follow applicable procedures for receiving, handling, administration, and disposal. |
|
MIMS Class
|
|
ATC Classification
L01XJ01 - vismodegib ; Belongs to the class of hedgehog pathway inhibitors. Used in the treatment of cancer.
|
|
References
Anon. Vismodegib (Briggs Drugs in Pregnancy and Lactation). Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 02/04/2018. Anon. Vismodegib. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 02/04/2018. Anon. Vismodegib. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 02/04/2018. Buckingham R (ed). Vismodegib. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 02/04/2018. Erivedge Capsules (Genentech, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 02/04/2018. Joint Formulary Committee. Vismodegib. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 02/04/2018. Preston CL (ed). Vismodegib Drug Interactions. Stockley’s Drug Interactions [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 13/04/2018.
|