Chronic hepatitis B
Adult: 600 mg once daily.
|
Indications and Dosage
Oral
Chronic hepatitis B Adult: 600 mg once daily.
|
||||||
|
Renal Impairment
ESRD: 600 mg 96 hrly, after dialysis session.
|
||||||
|
Administration
May be taken with or without food.
|
||||||
|
Contraindications
Hypersensitivity. Lactation. Concurrent use w/ peginterferon alfa-2a.
|
||||||
|
Special Precautions
Patient w/ cirrhosis, hepatomegaly or other risk factors for liver disease. Renal impairment. Pregnancy.
|
||||||
|
Adverse Reactions
Cough, dizziness, fatigue, GI effects (e.g. abdominal pain, diarrhoea, nausea, vomiting, dyspepsia), rash, arthralgia, myalgia, myopathy, malaise, back pain, nasopharyngitis, headache, flu or flu-like symptoms, insomnia; increased serum amylase, lipase, creatine phosphokinase, alanine aminotransferase levels; peripheral neuropathy, rhabdomyolysis.
Potentially Fatal: Lactic acidosis, severe hepatomegaly w/ steatosis. |
||||||
|
Monitoring Parameters
Monitor hepatic function (e.g. AST and ALT) periodically during therapy and for several mth following discontinuation of therapy; renal function; signs and symptoms of peripheral neuropathy or myopathy; serum creatine kinase; hepatitis B virus (HBV) DNA 3-6 mthly during therapy; HBeAg and anti-HBe signs/symptoms of HBV relapse/exacerbation after discontinuation of therapy.
|
||||||
|
Drug Interactions
Altered plasma concentration w/ drugs that affect renal function (e.g. aminoglycosides, loop diuretics, platinum compounds, vancomycin, amphotericin B). May increase risk of myopathy w/ other drugs associated w/ myopathy (e.g. azole antifungals, ciclosporin, corticosteroids, erythromycin, fibrates, HMG-CoA reductase inhibitors, penicillamine, zidovudine).
Potentially Fatal: Increased risk of peripheral neuropathy w/ peginterferon alfa-2a. |
||||||
|
Action
Description: Telbivudine is phosphorylated intracellularly to the active triphosphate form, which competes w/ thymidine 5'-triphosphate, the natural substrate of hepatitis B virus (HBV) reverse transcriptase, resulting to DNA chain termination and inhibition of HBV replication.
Pharmacokinetics: Absorption: Absorbed from the GI tract. Time to peak plasma concentration: Approx 3 hr. Distribution: Widely distributed into tissues. Plasma protein binding: Approx 3.3%. Metabolism: Undergoes phosphorylation by cellular kinases to form the active metabolite, telmivudine-5'-triphosphate. Excretion: Via urine, as unchanged drug. Terminal elimination half-life: 30-53.6 hr. |
||||||
|
Chemical Structure
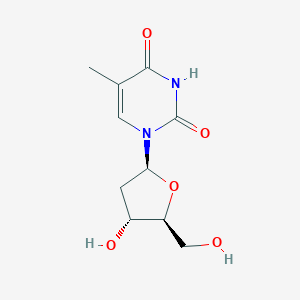 Source: National Center for Biotechnology Information. PubChem Database. Telbivudine, CID=159269, https://pubchem.ncbi.nlm.nih.gov/compound/Telbivudine (accessed on Jan. 23, 2020) |
||||||
|
Storage
Store at 25°C.
|
||||||
|
MIMS Class
|
||||||
|
ATC Classification
J05AF11 - telbivudine ; Belongs to the class of nucleoside and nucleotide reverse transcriptase inhibitors. Used in the systemic treatment of viral infections.
|
||||||
|
References
Anon. Telbivudine. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 21/09/2015. Anon. Telbivudine. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 21/09/2015. Buckingham R (ed). Telbivudine. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 21/09/2015. Tyzeka Film-Coated Tablet, Tyzeka Solution (Novartis Pharmaceuticals Corporation). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 21/09/2015.
|