Benign prostatic hyperplasia
Adult: 400 mcg once daily.
|
Indications and Dosage
Oral
Benign prostatic hyperplasia Adult: 400 mcg once daily.
|
|
Special Patient Group
Pharmacogenomics:
Tamsulosin is extensively metabolised in the liver by CYP3A4 and CYP2D6 into metabolites. Genetic polymorphism of CYP2D6 gene may affect the plasma concentrations of tamsulosin. The prevalence of CYP2D6 poor metabolisers is estimated in 7% of Caucasians and 2% of African Americans. Genetic testing may be considered prior to initiation of therapy. CYP2D6 poor metabolisers Since CYP2D6 poor metabolisers cannot be readily identified and the potential increase in tamsulosin exposure exists only when co-administered with CYP3A4 inhibitors in CYP2D6 poor metabolisers, tamsulosin should not be used with strong CYP3A4 inhibitors (e.g. ketoconazole). |
|
Renal Impairment
Severe: Contraindicated.
|
|
Hepatic Impairment
Severe: Contraindicated.
|
|
Administration
cap: Should be taken with food. Take 30 min following the same meal daily. Swallow whole, do not open/chew/crush.
prolonged release tab: May be taken with or without food. Swallow whole, do not chew/crush. orodispersible extended-release tab: Should be taken with food. Place on the tongue & allow to dissolve. Then, swallow w/ saliva or water. |
|
Contraindications
History of orthostatic hypotension. Severe renal impairment (CrCl <10 mL/min). Severe hepatic impairment (Child-Pugh scores >9). Concomitant use with strong CYP3A4 inhibitors in CYP2D6 poor metabolisers.
|
|
Special Precautions
Patient with myocardial dysfunction or angina pectoris; history of sulphonamide allergy; prostate cancer. Discontinue therapy 5-9 weeks prior to cataract or glaucoma surgery. Not indicated for use in women.
|
|
Adverse Reactions
Significant: 1st dose orthostatic hypotension, syncope, floppy iris syndrome in cataract and glaucoma surgery, hypersensitivity reactions, MI exacerbations.
Cardiac disorders: Dyspnoea, palpitations. Eye disorders: Visual impairment, blurred vision. Gastrointestinal disorders: Constipation, vomiting, dry mouth, diarrhoea, nausea. General disorders and admin site conditions: Malaise. Nervous system disorders: Headache, dizziness. Reproductive system and breast disorders: Ejaculation disorders including retrograde ejaculation, ejaculation failure, rarely, priapism. Respiratory, thoracic and mediastinal disorders: Rhinitis. Skin and subcutaneous tissue disorders: Pruritus, urticaria, skin desquamation including erythema multiforme, dermatitis exfoliative, rarely, Stevens-Johnson syndrome. Vascular disorders: Hypotension, epistaxis. |
|
Patient Counseling Information
This medicine may cause dizziness and syncope, if affected, do not drive or operate machinery.
|
|
Monitoring Parameters
Rule out prostate carcinoma with screening prior to initiation of therapy and screen at regular intervals. Monitor blood pressure and urinary symptoms. Perform digital rectal examination prior to treatment then regularly, thereafter.
|
|
Overdosage
Symptoms: Severe hypotensive effects, vomiting, diarrhoea. Management: Supportive and symptomatic treatment. CV support may be given in case of acute hypotension. Lie the patient down to restore blood pressure and to normalise heart rate. For severe cases, volume expanders and vasopressors may be given. Administer activated charcoal and osmotic laxative or perform gastric lavage for ingestion of large doses.
|
|
Drug Interactions
Increased serum concentration with weak or strong CYP3A4 inhibitors (e.g. cimetidine, ketoconazole) and strong CYP2D6 inhibitors (e.g. paroxetine). Decreased plasma levels with furosemide. Increased elimination rate with diclofenac and warfarin. Enhanced hypotensive effect with PDE5 inhibitors. May enhance the antihypertensive effect of other α1-adrenoceptor blockers.
|
|
Food Interaction
Food reduces rate and extent of absorption.
|
|
Action
Description: Tamsulosin is an antagonist of α1-adrenoceptors, which mediate smooth muscle tone in the prostate. This leads to the relaxation of smooth muscle in the bladder neck and prostate thereby improving urine flow and reducing the symptoms of benign prostatic hyperplasia (BPH).
Pharmacokinetics: Absorption: Rapidly absorbed from the gastrointestinal tract. Bioavailability: 30% increase in fasting state. Food reduces rate and extent of absorption. Time to peak plasma concentration: Approx 1 hour (immediate-release); approx 6 hours (prolonged-release). Distribution: Volume of distribution: Approx 0.2 L/kg (prolonged-release); 16 L (immediate-release). Plasma protein binding: Approx 99%, mainly to α1-acid glycoprotein. Metabolism: Extensively metabolised in the liver by CYP3A4 and CYP2D6 to metabolites and further undergoes extensive conjugation to glucuronide or sulfate. Excretion: Via urine (76%, <10% as unchanged drug); faeces (21%). Elimination half-life: 5-7 hours (immediate-release); 9-13 hours (prolonged-release). |
|
Chemical Structure
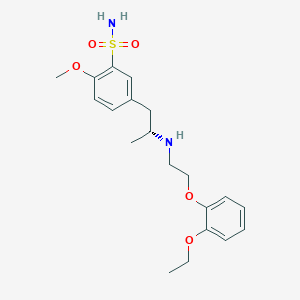 Source: National Center for Biotechnology Information. PubChem Database. Tamsulosin, CID=129211, https://pubchem.ncbi.nlm.nih.gov/compound/Tamsulosine (accessed on Jan. 23, 2020) |
|
Storage
Store below 30°C.
|
|
MIMS Class
|
|
ATC Classification
G04CA02 - tamsulosin ; Belongs to the class of alpha-adrenoreceptor antagonists. Used in the treatment of benign prostatic hypertrophy.
|
|
References
Annotation of FDA Label for Tamsulosin and CYP2D6. Pharmacogenomics Knowledgebase (PharmGKB). https://www.pharmgkb.org. Accessed 23/07/2019. Anon. Tamsulosin. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 23/07/2019. Buckingham R (ed). Tamsulosin. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 23/07/2019. Flomax Capsule (Boehringer Ingelheim Pharmaceuticals, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 13/11/2014. Flomax Capsule (Boehringer Ingelheim Pharmaceuticals, Inc.). U.S. FDA. https://www.fda.gov/. Accessed 23/07/2019. McEvoy GK, Snow EK, Miller J et al (eds). Tamsulosin Hydrochloride. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 13/11/2014. Tamsulosin Capsule (Sandoz Inc). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 23/07/2019.
|