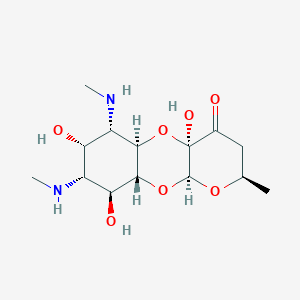
Gonorrhoea
Adult: 2 g as a single dose. In cases whereby antibiotic resistance is prevalent, 4 g (10 mL) may be given but divided as two 5 mL inj, admin to 2 different inj sites.
Child: Beyond newborn period and <45 kg: 40 mg/kg as a single dose. Max: 2 g.
Child: Beyond newborn period and <45 kg: 40 mg/kg as a single dose. Max: 2 g.




 Sign Out
Sign Out