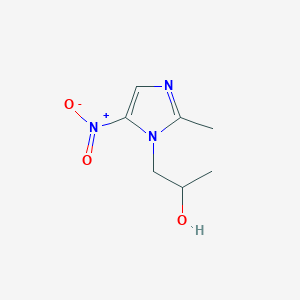
Intestinal amoebiasis
Adult: As tab: 2 g as single dose. Treatment recommendations may vary among countries and available products. Consider local treatment or detailed product guidelines on the appropriate use or recommendations.
Child: As tab: 30 mg/kg as single dose.
Child: As tab: 30 mg/kg as single dose.
Oral
Hepatic amoebiasis
Adult: As tab: 1.5 g daily as single or in divided doses for 5 days. Treatment recommendations may vary among countries and available products. Consider local treatment or detailed product guidelines on the appropriate use or recommendations.
Child: As tab: 30 mg/kg daily as single or in divided doses for 5 days.
Child: As tab: 30 mg/kg daily as single or in divided doses for 5 days.
Oral
Bacterial vaginosis
Adult: As granules or tab: 2 g as single dose. Treatment recommendations may vary among countries and available products. Consider local treatment or detailed product guidelines on the appropriate use or recommendations.
Oral
Trichomoniasis
Adult: As tab: 2 g as single dose. Treatment recommendations may vary among countries and available products. Consider local treatment or detailed product guidelines on the appropriate use or recommendations.
Oral
Giardiasis
Child: As tab: 30 mg/kg as single dose. Treatment recommendations may vary among countries and available products. Consider local treatment or detailed product guidelines on the appropriate use or recommendations.




 Sign Out
Sign Out