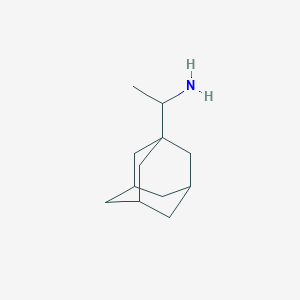
Prophylaxis of influenza A
Adult: 100 mg bid.
Child: 1-9 years 5mg/kg daily in 1 or 2 divided doses. Max: 150 mg daily. ≥10 years Same as adult dose.
Elderly: 100 mg daily.
Child: 1-9 years 5mg/kg daily in 1 or 2 divided doses. Max: 150 mg daily. ≥10 years Same as adult dose.
Elderly: 100 mg daily.
Oral
Influenza A
Adult: 100 mg bid for 7 days.
Elderly: 100 mg daily.
Elderly: 100 mg daily.




 Sign Out
Sign Out