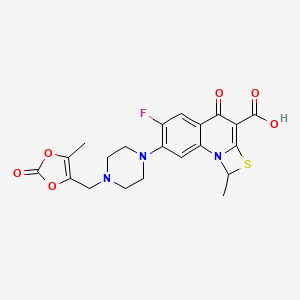
Acute uncomplicated urinary tract infections
Adult: 600 mg as a single dose. Treatment or dosage recommendations may vary among countries or individual products (refer to specific product or local treatment guidelines).
Oral
Acute exacerbations of chronic bronchitis, Complicated lower urinary tract infections
Adult: 600 mg once daily for up to a Max of 10 days, depending on the severity of the disease and clinical outcome. Treatment or dosage recommendations may vary among countries or individual products (refer to specific product or local treatment guidelines).




 Sign Out
Sign Out