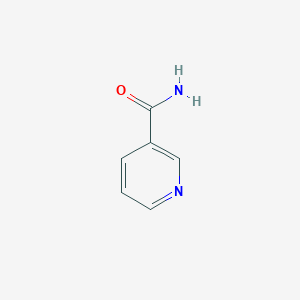
Treatment of nicotinic acid deficiency
Adult: 100-300 mg daily in divided doses.
Child: ≥12 yr Same as adult dose.
Child: ≥12 yr Same as adult dose.
Topical/Cutaneous
Mild to moderate inflammatory acne
Adult: As a 4% gel: Apply bid, reduce to once daily or on alternate days if excessive dryness, irritation or peeling occurs.
Child: Same as adult dose.
Child: Same as adult dose.




 Sign Out
Sign Out