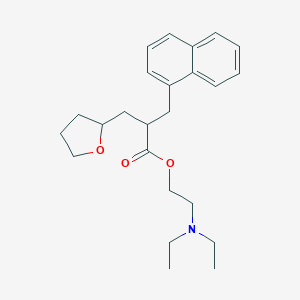
Peripheral vascular disease
Adult: For the treatment of cases such as intermittent claudication, night cramps, rest pain, Raynaud's syndrome, incipient gangrene, trophic ulcers, diabetic arteriopathy and acrocyanosis: 100-200 mg tid. Alternative recommended dose for Raynaud's syndrome: As tab: 200 mg bid. Dosing recommendations may vary among countries and individual products (refer to specific product guidelines).




 Sign Out
Sign Out