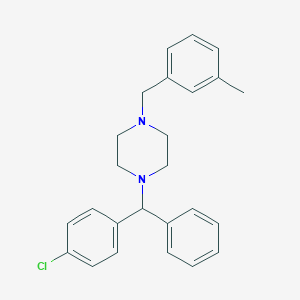
Vertigo
Adult: 25-100 mg daily in divided doses depending upon clinical response.
Child: ≥ 12 years Same as adult dose.
Child: ≥ 12 years Same as adult dose.
Oral
Motion sickness
Adult: 25-50 mg 1 hour before travel, repeat every 24 hours if necessary.
Child: 2-6 years 6.25 mg once daily; 6-12 years 12.5 mg once daily; >12 years Same as adult dose. All doses to be taken 1 hour before travel, repeat every 24 hours if necessary.
Child: 2-6 years 6.25 mg once daily; 6-12 years 12.5 mg once daily; >12 years Same as adult dose. All doses to be taken 1 hour before travel, repeat every 24 hours if necessary.




 Sign Out
Sign Out