Hypertension
Adult: Each tab contains irbesartan (mg)/hydrochlorothiazide (mg): 150/12.5, 300/12.5, or 300/25: Initially, 150 mg/12.5 mg once daily. If inadequate response, titrate dose after 1-2 wk. Max: 300 mg/25 mg once daily.
|
Indications and Dosage
Oral
Hypertension Adult: Each tab contains irbesartan (mg)/hydrochlorothiazide (mg): 150/12.5, 300/12.5, or 300/25: Initially, 150 mg/12.5 mg once daily. If inadequate response, titrate dose after 1-2 wk. Max: 300 mg/25 mg once daily.
|
||||
|
Renal Impairment
|
||||
|
Hepatic Impairment
Severe: Contraindicated.
|
||||
|
Administration
May be taken with or without food.
|
||||
|
Contraindications
Hypersensitivity to irbesartan, hydrochlorothiazide, or sulphonamide-derived drugs, refractory hypokalaemia, hypercalcaemia; biliary cirrhosis and cholestatis. Severe hepatic and renal (<30 mL/min) impairment. Pregnancy. Concomitant use w/ aliskiren-containing products in patients w/ DM or renal (GFR <60 mL/min) impairment.
|
||||
|
Special Precautions
Patient w/ aortic or mitral stenosis, obstructive hypertrophic cardiomyopathy, severe CHF, DM, hypercholesterolemia, parathyroid disease, renal artery stenosis, history of allergy or bronchial asthma. Mild to moderate hepatic and renal impairment. Lactation.
|
||||
|
Adverse Reactions
Significant: Hypo- or hyperkalaemia, hyponatremia, hypomagnesimia, hypochloremic alkalosis, hypotension, myopia, acute angle-closure glaucoma, SLE, hypersensitivity reactions (e.g. angioedema), gout, renal function deterioration, photosensitivity.
Nervous: Headache, flu-like symptoms, dizziness (including orthostatic dizziness), fatigue, anxiety, syncope. CV: Chest pain, oedema, tachycardia, flushing, decreased BP. GI: Diarrhoea, dyspepsia, dysgeusia, heartburn, nausea, vomiting, abdominal pain. Resp: Cough, pharyngitis, rhinitis. Hepatic: Jaudince, hepatitis, abnormal liver function. Genitourinary: Difficulty in micturition, UTI, elevated BUN and creatinine. Musculoskeletal: Musculoskeletal pain, arthralgia, increased creatine kinase. Otic: Tinnitus. |
||||
|
Patient Counseling Information
This drug may cause occasional dizziness and weariness, if affected, do not drive or operate machinery.
|
||||
|
Monitoring Parameters
Assess wt, input & output, BP, symptomatic hypotension and tachycardia, serum electrolytes, BUN, creatinine.
|
||||
|
Overdosage
Symptoms: Hypotension, tachycardia, bradycardia, electrolyte depletion, dehydration, nausea, somnolence, muscle spasm. Management: Symptomatic and supportive treatment. Employ activated charcoal or gastric lavage, and induce emesis. Treat hypotension w/ salt and volume replacements and monitor serum electrolytes and creatinine levels frequently.
|
||||
|
Drug Interactions
May increase serum lithium levels and toxicity. Enhanced hypotensive effect w/ other antihypertensive agents and barbiturates. Increased risk of hyperkalaemia w/ K-sparing diuretics, K supplements, or K-containing salt substitutes. Increased risk of symptomatic hyponatremia w/ carbamazepine. Irbesartan may cause acute renal failure w/ NSAIDs. Impaired absorption of hydrochlorothiazide w/ bile acid sequesterants (e.g. cholestyramine, colestipol). Hydrochlorothiazide may potentiate the effect of nondepolarising skeletal muscle relaxants (e.g. tubocurarine). Hydrochlorothiazide may enhance the hypercalcaemic effect of fat-soluble vit, folate, and Fe. Hydrochlorothiazide may increase electrolyte depletion, particularly hypokalaemia, when used w/ corticosteroids and ACTH. Thiazides may reduce the excretion of cytotoxic agents (e.g. cyclophosphamide, methotrexate) and potentiate their myelosuppressive effects. Thiazides may enhance the hyperglycaemic effect of β-blockers and diazoxide. Increased bioavailability of thiazides w/ anticholinergic agents (e.g. atropine, biperiden).
Potentially Fatal: Increased risk of hypotension, hyperkalaemia, and decreased renal function (e.g. acute renal failure) when concomitantly used w/ aliskiren esp in patients w/ DM or renal impairment. |
||||
|
Food Interaction
Diminished antihypertensive effect w/ yohimbine. Potentiated orthostatic hypotensive effect w/ alcohol.
|
||||
|
Lab Interference
May cause false-negative aldosterone/renin ratio (ARR). Hydrochlorothiazide may cause false-positive analytic result w/ anti-doping. Hydrochlorothiazide may interfere w/ parathyroid function test and decrease serum iodine w/out signs of thyroid disturbances.
|
||||
|
Action
Description: Irbesartan is a nonpeptide tetrazole derivative that blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively binding to AT1 receptors. Hydrochlorothiazide is a diuretic acting mainly at the beginning of the distal tubules. It increases the excretion of Na and Cl ions, and consequently of water, by reducing electrolyte reabsorption from the renal tubules.
Pharmacokinetics: Absorption: Irbesartan: Rapidly and completely absorbed from the GI tract. Bioavailability: 60-80%. Time to peak plasma concentration: 1.5-2 hr. Hydrochlorothiazide: Well absorbed from the GI tract. Bioavailability: 65-75%. Time to peak plasma concentration: Approx 1-2.5 hr. Distribution: Irbesartan: Volume of distribution: 53-93 L. Plasma protein-binding: Approx 96%, primarily to albumin and α-1 acid glycoprotein. Hydrochlorothiazide: Crosses the placenta and enters breast milk. Volume of distribution: 0.83-1.14 L/kg. Plasma protein binding: Approx 40-68%. Metabolism: Irbesartan: Metabolised in the liver via glucuronide conjugation and oxidation by CYP2C9 enzyme. Excretion: Irbesartan: Via faeces (80%) and urine (20%, <2% as unchanged drug). Terminal elimination half-life: 11-15 hr. Hydrochlorothiazide: Via urine, ≥61% as unchanged drug. Elimination half-life: Approx 6-15 hr. |
||||
|
Chemical Structure
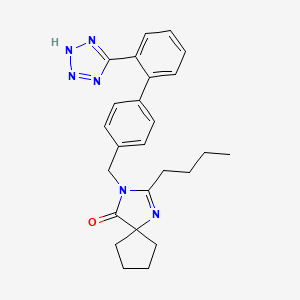 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 3749, Irbesartan. https://pubchem.ncbi.nlm.nih.gov/compound/Irbesartan. Accessed Oct. 26, 2022. 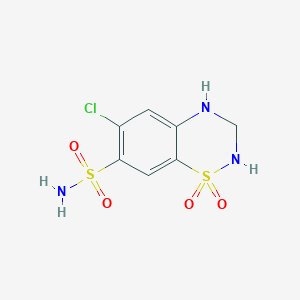 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 3639, Hydrochlorothiazide. https://pubchem.ncbi.nlm.nih.gov/compound/3639. Accessed Aug. 25, 2022. |
||||
|
Storage
Store at 25°C. Protect from moisture.
|
||||
|
MIMS Class
|
||||
|
ATC Classification
C09DA04 - irbesartan and diuretics ; Belongs to the class of angiotensin II receptor blockers (ARBs) in combination with diuretics. Used in the treatment of cardiovascular disease.
|
||||
|
References
Anon. Hydrochlorothiazide. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 09/06/2017. Anon. Irbesartan and Hydrochlorothiazide. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 08/06/2017. Anon. Irbesartan. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 08/06/2017. Anon. Irbesartan. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 08/06/2017. Anon. Thiazides General Statement. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 09/06/2017. Buckingham R (ed). Hydrochlorothiazide. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 08/06/2017. Buckingham R (ed). Irbesartan. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 08/06/2017. Irbesartan and Hydrochlorothiazide Tablet (Alembic Pharmaceuticals Limited). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 08/06/2017. Joint Formulary Committee. Irbesartan with Hydrochlorothiazide. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 08/06/2017.
|