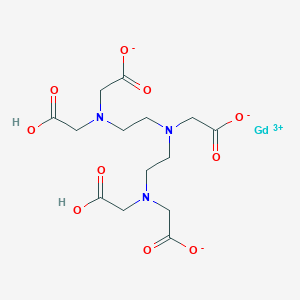
Cranial, spinal and whole body imaging
Adult: As gadopentetate dimeglumine: 0.1 mmol/kg (0.2 mL/kg), given at a rate not exceeding 10 mL/15 sec. If needed, an additional dose of 0.1-0.2 mmol/kg over 30 minutes may be given. Max: 0.3 mmol/kg.
Child: ≥1 month As gadopentetate dimeglumine: 0.1 mmol/kg (0.2 mL/kg), given at a rate not exceeding 10 mL/15 sec; >1 year If needed, additional dose of 0.1-0.2 mmol/kg over 30 minutes may be given.
Child: ≥1 month As gadopentetate dimeglumine: 0.1 mmol/kg (0.2 mL/kg), given at a rate not exceeding 10 mL/15 sec; >1 year If needed, additional dose of 0.1-0.2 mmol/kg over 30 minutes may be given.




 Sign Out
Sign Out