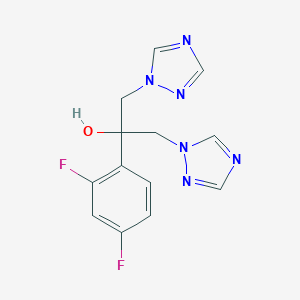
Oropharyngeal candidiasis
Adult: Treatment: Initially, 200-400 mg on Day 1, followed by 100-200 mg once daily for 7-21 days (until disease is in remission). Longer periods may be used in patients with severely compromised immune function. Prevention of relapse in patients with HIV: 100-200 mg once daily, or 200 mg 3 times a week. Infusion rate: 10 mL/min.
Child: 0-14 days Initially, 6 mg/kg, followed by 3 mg/kg every 72 hours. Max: 12 mg/kg 72 hourly. 15-27 days Initially, 6 mg/kg, followed by 3 mg/kg every 48 hours. Max: 12 mg/kg 48 hourly. 28 days-11 years Initially, 6 mg/kg, followed by 3 mg/kg once daily. Infusion rate: 10 mL/min.
Child: 0-14 days Initially, 6 mg/kg, followed by 3 mg/kg every 72 hours. Max: 12 mg/kg 72 hourly. 15-27 days Initially, 6 mg/kg, followed by 3 mg/kg every 48 hours. Max: 12 mg/kg 48 hourly. 28 days-11 years Initially, 6 mg/kg, followed by 3 mg/kg once daily. Infusion rate: 10 mL/min.
Intravenous
Oesophageal candidiasis
Adult: Treatment: Initially, 200-400 mg on Day 1, followed by 100-200 mg once daily for 14-30 days (until disease is in remission). Longer periods may be used in patients with severely compromised immune function. Prevention of relapse in patients with HIV: 100-200 mg once daily, or 200 mg 3 times a week. Infusion rate: 10 mL/min.
Child: 0-14 days Initially, 6 mg/kg, followed by 3 mg/kg every 72 hours. Max: 12 mg/kg 72 hourly. 15-27 days Initially, 6 mg/kg, followed by 3 mg/kg every 48 hours. Max: 12 mg/kg 48 hourly. 28 days-11 years Initially, 6 mg/kg, followed by 3 mg/kg once daily. Infusion rate: 10 mL/min.
Child: 0-14 days Initially, 6 mg/kg, followed by 3 mg/kg every 72 hours. Max: 12 mg/kg 72 hourly. 15-27 days Initially, 6 mg/kg, followed by 3 mg/kg every 48 hours. Max: 12 mg/kg 48 hourly. 28 days-11 years Initially, 6 mg/kg, followed by 3 mg/kg once daily. Infusion rate: 10 mL/min.
Intravenous
Coccidioidomycosis
Adult: 200-400mg once daily for 11-24 months, or longer depending on severity of infection, clinical or mycologic response. 800 mg may be considered for meningeal disease or some infections. Infusion rate: 10 mL/min.
Intravenous
Invasive candidiasis
Adult: Initially, 800 mg on Day 1, followed by 400 mg once daily for 2 weeks. Infusion rate: 10 mL/min.
Child: ≥4 weeks-11 years 6-12 mg/kg once daily. Infusion rate: 10 mL/min.
Child: ≥4 weeks-11 years 6-12 mg/kg once daily. Infusion rate: 10 mL/min.
Intravenous
Cryptococcal meningitis
Adult: Initially, 400 mg on Day 1, followed by 200-400 mg once daily, for at least 6-8 weeks. Treatment durations are based on severity of infection, clinical and mycologic response. Prevention of relapse in patients with high risk of recurrence: 200 mg once daily. Infusion rate: 10 mL/min.
Child: ≥4 weeks-11 years Treatment: 6-12 mg/kg once daily. Prevention of relapse in patients with high risk of recurrence: As maintenance therapy, 6 mg/kg once daily. Infusion rate: 10 mL/min.
Child: ≥4 weeks-11 years Treatment: 6-12 mg/kg once daily. Prevention of relapse in patients with high risk of recurrence: As maintenance therapy, 6 mg/kg once daily. Infusion rate: 10 mL/min.
Intravenous
Chronic atrophic candidiasis
Adult: 50 mg once daily for 14 days. Infusion rate: 10 mL/min.
Intravenous
Candiduria
Adult: 200-400 mg once daily for 7-21 days. Longer periods may be used in patients with severely compromised immune function. Infusion rate: 10 mL/min.
Intravenous
Chronic mucocutaneous candidiasis
Adult: 50-100 mg once daily for up to 28 days. Longer periods depending on both the severity of infection or underlying compromised immune function and infection. Infusion rate: 10 mL/min.
Intravenous
Prophylaxis of fungal infections in immunocompromised patients
Adult: 200-400 mg once daily. Start treatment before anticipated onset of neutropenia and continue for 7 days after neutrophil count is within desirable range. Infusion rate: 10 mL/min.
Child: ≥4 weeks-11 years 3-12 mg/kg once daily. Infusion rate: 10 mL/min.
Child: ≥4 weeks-11 years 3-12 mg/kg once daily. Infusion rate: 10 mL/min.
Oral
Oropharyngeal candidiasis
Adult: Treatment: Initially, 200-400 mg on Day 1, followed by 100-200 mg once daily for 7-21 days (until disease is in remission). Longer periods may be used in patients with severely compromised immune function. Prevention of relapse in patients with HIV: 100-200 mg once daily, or 200 mg 3 times a week.
Child: 0-14 days Initially, 6 mg/kg, followed by 3 mg/kg every 72 hours. Max: 12 mg/kg 72 hourly. 15-27 days Initially, 6 mg/kg, followed by 3 mg/kg every 48 hours. Max: 12 mg/kg 48 hourly. 28 days-11 years Initially, 6 mg/kg, followed by 3 mg/kg once daily.
Child: 0-14 days Initially, 6 mg/kg, followed by 3 mg/kg every 72 hours. Max: 12 mg/kg 72 hourly. 15-27 days Initially, 6 mg/kg, followed by 3 mg/kg every 48 hours. Max: 12 mg/kg 48 hourly. 28 days-11 years Initially, 6 mg/kg, followed by 3 mg/kg once daily.
Oral
Oesophageal candidiasis
Adult: Treatment: Initially, 200-400 mg on Day 1, followed by 100-200 mg once daily for 14-30 days (until disease is in remission). Longer periods may be used in patients with severely compromised immune function. Prevention of relapse in patients with HIV: 100-200 mg once daily, or 200 mg 3 times a week.
Child: 0-14 days Initially, 6 mg/kg, followed by 3 mg/kg every 72 hours. Max: 12 mg/kg 72 hourly. 15-27 days Initially, 6 mg/kg, followed by 3 mg/kg every 48 hours. Max: 12 mg/kg 48 hourly. 28 days-11 years Initially, 6 mg/kg, followed by 3 mg/kg once daily.
Child: 0-14 days Initially, 6 mg/kg, followed by 3 mg/kg every 72 hours. Max: 12 mg/kg 72 hourly. 15-27 days Initially, 6 mg/kg, followed by 3 mg/kg every 48 hours. Max: 12 mg/kg 48 hourly. 28 days-11 years Initially, 6 mg/kg, followed by 3 mg/kg once daily.
Oral
Candiduria
Adult: 200-400 mg once daily for 7-21 days. Longer periods may be used in patients with severely compromised immune function.
Oral
Chronic mucocutaneous candidiasis
Adult: 50-100 mg once daily for up to 28 days. Longer periods depending on both the severity of infection or underlying compromised immune function and infection.
Oral
Chronic atrophic candidiasis
Adult: 50 mg once daily for 14 days.
Oral
Dermatophytosis
Adult: Tinea pedis, Tinea corporis, Tinea cruris, Candida infections: 150 mg once a week, or 50 mg once daily. Tinea unguium (onychomycosis): 150 mg once weekly. Treatment durations are based on severity of infection, clinical or mycologic response. Tinea versicolor: 300-400 mg once a week for 1-3 weeks, or 50 mg once daily for 2-4 weeks.
Oral
Candidal balanitis, Vaginal candidiasis
Adult: 150 mg given as a single dose. Treatment and prophylaxis of recurrent vaginal candidiasis (4 or more episodes a year): 150 mg every third day for a total of 3 doses (Day 1, 4, and 7), followed by a 150 mg once weekly maintenance dose (6 months).
Oral
Coccidioidomycosis
Adult: 200-400 mg once daily for 11-24 months, or longer depending on severity of infection, clinical or mycologic response. 800 mg may be considered for meningeal disease or some infections.
Oral
Prophylaxis of fungal infections in immunocompromised patients
Adult: In patients with prolonged neutropenia: 200-400 mg once daily. Start treatment before anticipated onset of neutropenia and continue for 7 days after neutrophil count is within desirable range.
Child: ≥4 weeks-11 years 3-12 mg/kg once daily.
Child: ≥4 weeks-11 years 3-12 mg/kg once daily.
Oral
Cryptococcal meningitis
Adult: Initially, 400 mg on Day 1, followed by 200-400 mg once daily, for at least 6-8 weeks. Treatment durations are based on severity of infection, clinical and mycologic response. Prevention of relapse in patients with high risk of recurrence: 200 mg once daily.
Child: ≥4 weeks-11 years Treatment: 6-12 mg/kg once daily. Prevention of relapse in patients with high risk of recurrence: As maintenance therapy, 6 mg/kg once daily.
Child: ≥4 weeks-11 years Treatment: 6-12 mg/kg once daily. Prevention of relapse in patients with high risk of recurrence: As maintenance therapy, 6 mg/kg once daily.
Oral
Invasive candidiasis
Adult: Initially, 800 mg on Day 1, followed by 400 mg once daily for 2 weeks.
Child: ≥4 weeks-11 years 6-12 mg/kg once daily.
Child: ≥4 weeks-11 years 6-12 mg/kg once daily.




 Sign Out
Sign Out