OralChronic pain, Severe painAdult: As conventional tab: 40-80 mg tid. As prolonged-release tab: 60-120 mg 12 hourly. Max: 240 mg daily.
Child: >12 years Same as adult dose.
Elderly: Dose reduction may be needed.
OralModerate to severe painAdult: As immediate-release preparation: 30 mg 4-6 hourly.
Child: 4-12 years 0.5-1 mg/kg every 4-6 hours. >12 years Same as adult dose.
Elderly: Dose reduction may be needed.
OralCough suppressant, Non-productive coughAdult: 10 mg 4-6 hourly.
Child: 4-12 years 0.2 mg/kg every 4-6 hours. >12 years Same as adult dose.
Elderly: Dose reduction may be needed.
ParenteralSevere painAdult: 50 mg 4-6 hourly via deep SC or IM inj.
Child: ≥4 years 0.5-1 mg/kg 4-6 hourly via deep SC or IM inj.
Elderly: Dose reduction may be needed.
|
Dose reduction may be needed.
|
Dose reduction may be needed.
|
Should be taken with food.
|
Acute or severe respiratory depression, chronic obstructive airways disease, severe cor pulmonale, phaeochromocytoma, acute asthma attack, infective bronchial condition, risk of paralytic ileus, acute diarrhoeal condition (e.g. acute ulcerative colitis, antibiotic-associated colitis), raised intracranial pressure or head injury, heart failure secondary to lung disease, acute alcoholism, biliary tract disorders, comatose state. Children below 4 years. Concomitant or within 2 weeks of MAOI use.
|
Patient with asthma, adrenocortical insufficiency, prostatic hyperplasia, urethral stricture, hypotension, shock, inflammatory or obstructive bowel disorders, myasthenia gravis, hypothyroidism, history of drug abuse or dependence, depressed respiratory reserve, convulsive disorders. Renal and hepatic impairment. Pregnancy and lactation. Treatment with cough medicine in children should be considered carefully due to potential risks and limited evidence on efficacy. Elderly.
|
Significant: Drowsiness, dependence and tolerance, increased serum prolactin, decreased plasma cortisol and testosterone.
Gastrointestinal disorders: Abdominal pain, constipation, dry mouth, nausea, vomiting.
Nervous system disorders: Somnolence, headache, vertigo.
Psychiatric disorders: Confusion, hallucinations, dysphoria, mood changes.
Renal and urinary disorders: Difficulty in micturition.
Skin and subcutaneous tissue disorders: Sweating, miosis, facial flushing, rashes, pruritus.
Vascular disorders: Postural hypotension.
Potentially Fatal: Respiratory depression.
|
This drug may cause drowsiness and visual changes (e.g. blurred vision, double vision), if affected, do not drive or operate machinery.
|
Symptoms: Pin point pupil, respiratory depression or coma. Hypothermia, confusion, convulsions, severe dizziness and drowsiness, hypotension, nervousness or restlessness, hallucinations, slow heart rate, circulatory failure, slow or troubled breathing, severe weakness, rhabdomyolysis. Management: May administer opioid antagonist (e.g. naloxone) IV as required. If substantial amount has been ingested within 1 hour, consider activated charcoal (provided the airway can be protected). If prolonged-release formulation has been taken, empty stomach via gastric lavage.
|
May delay absorption of mexiletine. May enhanced the CNS depressant effects of anxiolytics (e.g. diazepam, chlordiazepoxide), hypnotics, antipsychotics, and TCAs. Enhanced respiratory/CNS depression and hypotension with anaesthetics, antihistamines and Na oxybate. Increased risk of severe constipation with loperamide and kaolin. May antagonise the effect of cisapride, metoclopramide and domperidone. Increased plasma concentration with cimetidine.
Potentially Fatal: Enhanced respiratory depressant effect with MAOIs.
|
Enhanced sedative effect with alcohol.
|
May interfere with gastric-emptying studies and hepatobiliary imaging tests using technetium Tc 99m disofenin.
|
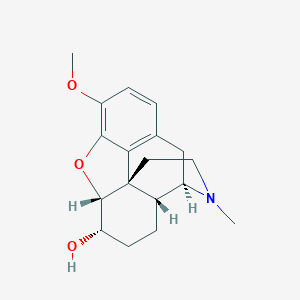
Description: Dihydrocodeine is an opioid derivative. It acts on opioid receptors in the brain to reduce perception to pain and improves psychological reactions to pain by reducing anxiety. It can also be used as cough suppressant by depressing the medullary cough centre.
Pharmacokinetics:
Absorption: Bioavailability: Approx 20%. Time to peak plasma concentration: Approx 1.2-1.8 hours.
Distribution: Well absorbed (oral).
Metabolism: Undergoes first-pass metabolism in the gut wall or liver. Metabolised by CYP2D6 to dihydromorphine and by CYP3A4 to nordihydrocodeine.
Excretion: Via urine as unchanged drug and metabolites including glucuronide conjugates. Elimination half-life: Approx 3.5 hours.
|
|
|
Store below 25°C. Protect from light.
|
|
|
N02AA08 - dihydrocodeine ; Belongs to the class of natural opium alkaloids. Used to relieve pain.
|
DF118 Forte 40 mg (Martindale Pharmaceuticals Ltd.). MHRA. https://products.mhra.gov.uk/ . Accessed 06/08/2018. Joint Formulary Committee. Dihydrocodeine Tartrate. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 06/08/2018. Pharmaco NZ Ltd. DHC Continus 60 mg Tablets data sheet July 2017. Medsafe. http://www.medsafe.govt.nz/. Accessed 06/08/2018.
|




 Sign Out
Sign Out