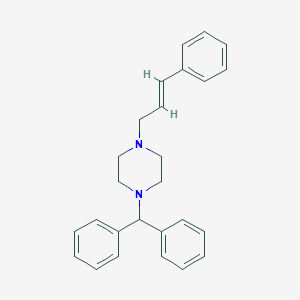
Labyrinthine disorder
Adult: For symptoms of labyrinthine disorders, including dizziness, vertigo, tinnitus, nystagmus, nausea and vomiting: As tab: 25 mg or 30 mg tid. As cap: 75 mg once daily. Dosage recommendations may vary among individual products or between countries (refer to detailed product guidelines).
Child: As 15 mg tab: 5-12 years 15 mg tid; >12 years Same as adult dose.
Child: As 15 mg tab: 5-12 years 15 mg tid; >12 years Same as adult dose.
Oral
Cerebrovascular disorders
Adult: As cap: 75 mg once daily.
Oral
Prophylaxis of motion sickness
Adult: As 15 mg tab: Initially, 30 mg to be taken 2 hours before travel, then 15 mg 8 hourly during the journey if necessary. As 25 mg tab: 25 mg to be taken at least 30 minutes before travel, may be repeated 6 hourly if necessary. Dosage recommendations may vary among individual products or between countries (refer to detailed product guidelines).
Child: As 15 mg tab: 5-12 years Initially, 15 mg to be taken 2 hours before travel, then 7.5 mg 8 hourly during the journey if necessary; >12 years Same as adult dose. As 25 mg tab: 6-12 years 12.5 mg to be taken at least 30 minutes before travel, may be repeated 6 hourly if necessary; ≥13 years Same as adult dose. Dosage recommendations may vary among individual products or between countries (refer to detailed product guidelines).
Child: As 15 mg tab: 5-12 years Initially, 15 mg to be taken 2 hours before travel, then 7.5 mg 8 hourly during the journey if necessary; >12 years Same as adult dose. As 25 mg tab: 6-12 years 12.5 mg to be taken at least 30 minutes before travel, may be repeated 6 hourly if necessary; ≥13 years Same as adult dose. Dosage recommendations may vary among individual products or between countries (refer to detailed product guidelines).
Oral
Peripheral circulatory disorders
Adult: As 25 mg tab: 50-75 mg 2-3 times daily. Max: 225 mg daily.




 Sign Out
Sign Out