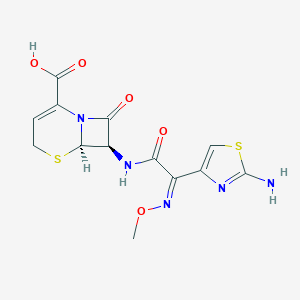
Uncomplicated gonorrhoea
Adult: 1 g as a single dose.
Parenteral
Uncomplicated urinary tract infections
Adult: 0.5 g 12 hrly given as deep IM or slow IV inj over 3-5 min.
Parenteral
Susceptible infections
Adult: 1-2 g 8-12 hrly given as deep IM or slow IV inj over 3-5 min, increased to 2-4 g IV 8 hrly in severe infections. Max: 2 g 4 hrly.
Child: ≥6 mth 50 mg/kg 6-8 hrly.
Child: ≥6 mth 50 mg/kg 6-8 hrly.




 Sign Out
Sign Out