Allergic conditions
Adult: 4 mg 4-6 hrly.
Child: 2-6 yr 1 mg 4-6 hrly; >6-12 yr 2 mg 4-6 hrly; >12 yr Same as adult dose.
Child: 2-6 yr 1 mg 4-6 hrly; >6-12 yr 2 mg 4-6 hrly; >12 yr Same as adult dose.
|
Indications and Dosage
Oral
Allergic conditions Adult: 4 mg 4-6 hrly.
Child: 2-6 yr 1 mg 4-6 hrly; >6-12 yr 2 mg 4-6 hrly; >12 yr Same as adult dose. |
|
Administration
May be taken with or without food. May be taken w/ food or milk to reduce GI discomfort.
|
|
Incompatibility
Incompatible w/ some amidotrizoate, adipiodone and iotalamate salts.
|
|
Contraindications
Pregnancy (3rd trimester).
|
|
Special Precautions
Patient w/ glaucoma, prostatic hyperplasia, genitourinary obstruction, asthma, chronic bronchitis, thyroid dysfunction, CV disease (e.g. HTN, ischaemic heart disease). Childn. Pregnancy and lactation.
|
|
Adverse Reactions
Excitability (esp in childn), drowsiness, sedation, angina pectoris, chest tightness, circulatory shock, extrasystoles, hypotension, palpitation, increased BP, tachycardia, anxiety, ataxia, chills, confusion, euphoria, fatigue, headache, hysteria, insomnia, irritability, nervousness, neuritis, paraesthesia, restlessness, seizure, tension, vertigo, diaphoresis, photosensitivity, rash, urticaria, abdominal cramps, anorexia, constipation, diarrhoea, epigastric distress, heartburn, nausea, vomiting, xerostomia, dysuria, early menstruation, urinary retention, agranulocytosis, haemolytic anaemia, thrombocytopenia, tremor, weakness, diplopia, mydriasis, blurred vision, acute labyrinthitis, tinnitus, polyuria, dry nose or throat, nasal congestion, bronchial secretions, wheezing.
|
|
Patient Counseling Information
This drug may cause drowsiness and sedation, if affected, do not drive or operate machinery.
|
|
Drug Interactions
May enhance the sedative effects of CNS depressants (e.g. barbiturates, anxiolytics, hypnotics, opioid analgesics, antipsychotics). Additive antimuscarinic effects w/ other antimuscarinic drugs (e.g. atropine, TCAs, MAOIs). May mask the warning signs of damage caused by ototoxic drugs (e.g. aminoglycosides).
|
|
Food Interaction
May enhance the CNS depressant effects of alcohol.
|
|
Lab Interference
May cause false-positive result w/ urine detection of amphetamine/methamphetamine. May interfere w/ skin tests using allergen extracts.
|
|
Action
Description: Brompheniramine is an antihistamine w/ antimuscarinic and moderate sedative actions. It competes w/ histamine for H1-receptor sites on effector cells.
Pharmacokinetics: Absorption: Well absorbed from the GI tract. Time to peak plasma concentration: 2-4 hr. Distribution: Widely distributed. Volume of distribution: Approx 12 L/kg. Plasma protein binding: 39-49%. Metabolism: Undergoes N-dealkylation to form monodesmethylbrompheniramine and didesmethylbrompheniramine, and is metabolised to propionic acid derivative. Excretion: Via urine (approx 40%, as unchanged drug and metabolites) and faeces (approx 2%). Elimination half-life: Approx 25 hr. |
|
Chemical Structure
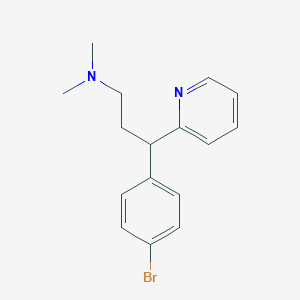 Source: National Center for Biotechnology Information. PubChem Database. Brompheniramine, CID=6834, https://pubchem.ncbi.nlm.nih.gov/compound/Brompheniramine (accessed on Jan. 22, 2020) |
|
Storage
Store between 15-30°C. Protect from light.
|
|
MIMS Class
|
|
ATC Classification
R06AB01 - brompheniramine ; Belongs to the class of substituted alkylamines used as systemic antihistamines.
|
|
References
Anon. Brompheniramine. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 23/08/2016. Buckingham R (ed). Brompheniramine maleate. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 23/08/2016. Buckingham R (ed). Interactions of Antihistamines . Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 23/09/2016 . J-TAN PD Liquid (JayMac Pharmaceuticals LLC). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 23/08/2016. McEvoy GK, Snow EK, Miller J et al (eds). Brompheniramine Maleate, Dexbrompheniramine Maleate . AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 23/08/2016.
|