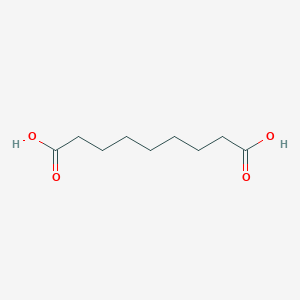
Acne vulgaris
Adult: As 20% cream or 15% gel: Apply thinly into the affected areas bid (morning and evening) after cleansing. Improvement may be detectable w/n 4 wk. Duration of treatment: Up to 6 mth.
Child: ≥12 yr Same as adult dose.
Child: ≥12 yr Same as adult dose.
Topical/Cutaneous
Rosacea
Adult: As 15% gel: Apply thinly into the affected areas bid (morning and evening). Improvement occurs in 4-8 wk.
Child: ≥12 yr Same as adult dose.
Child: ≥12 yr Same as adult dose.




 Sign Out
Sign Out