Chronic hepatitis B
Adult: 10 mg once daily.
|
Indications and Dosage
Oral
Chronic hepatitis B Adult: 10 mg once daily.
|
||||||
|
Renal Impairment
Haemodialysis patients: 10 mg every 7 days after dialysis.
|
||||||
|
Contraindications
Hypersensitivity. Lactation.
|
||||||
|
Special Precautions
Patient w/ hepatomegaly or other risk factors for liver disease. Renal impairment. Pregnancy.
|
||||||
|
Adverse Reactions
Nephrotoxicity (chronic use); GI effects (e.g. nausea, flatulence, diarrhoea, dyspepsia, vomiting, abdominal pain, pancreatitis), headache, asthenia, pruritus, skin rash, proximal renal tubulopathy, Fanconi syndrome, hypophosphatemia, raised liver enzymes and serum creatinine concentrations, renal failure, renal insufficiency, myopathy, osteomalacia.
Potentially Fatal: Lactic acidosis, severe hepatomegaly w/ steatosis. |
||||||
|
Monitoring Parameters
Hepatitis B biochemical, virological and serological markers should be monitored 6 mthly. Monitor renal function 3 mthly, hepatic function for several mth following discontinuation. Determine HIV status prior to treatment.
|
||||||
|
Overdosage
Symptoms: GI disorders, anorexia. Management: Supportive treatment. Haemodialysis can remove adefovir from the body.
|
||||||
|
Drug Interactions
May diminish the therapeutic effect of tenofovir. Increased risk of nephrotoxicity w/ other nephrotoxic drugs (e.g. aminoglycosides, ciclosporin, tacrolimus, vancomyicn, certain NSAIDs).
|
||||||
|
Food Interaction
Delayed absorption when given w/ food.
|
||||||
|
Action
Description: Adefovir, an acyclic nucleotide analogue of adenosine monophosphate is phosphorylated to the active metabolite adefovir diphosphate, which then inhibits hepatitis B virus (HBV) DNA polymerase (reverse transcriptase) by competing w/ the natural substrate deoxyadenosine triphosphate and by causing DNA chain termination after its incorporation into viral DNA, thus resulting to inhibition of viral replication.
Pharmacokinetics: Absorption: Delayed absorption when given w/ food. Bioavailability: 59%. Time to peak plasma concentration: Approx 0.6-4 hr. Distribution: Widely distributed to body tissues particularly in kidneys, liver and intestines. Volume of distribution: 0.35-0.39 L/kg. Plasma protein binding: <4%. Metabolism: Adefovir dipivoxil is rapidly converted to adefovir via diester hydrolysis and subsequently, to the active metabolite adefovir diphosphate via phosphorylation by cellular enzymes. Excretion: Via urine by glomerular filtration and active tubular secretion. Terminal elimination half-life: Approx 7 hr. |
||||||
|
Chemical Structure
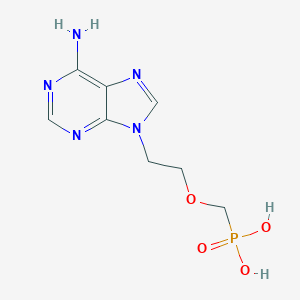 Source: National Center for Biotechnology Information. PubChem Database. Adefovir, CID=60172, https://pubchem.ncbi.nlm.nih.gov/compound/Adefovir (accessed on Jan. 20, 2020) |
||||||
|
Storage
Store at 25°C.
|
||||||
|
MIMS Class
|
||||||
|
ATC Classification
J05AF08 - adefovir dipivoxil ; Belongs to the class of nucleoside and nucleotide reverse transcriptase inhibitors. Used in the systemic treatment of viral infections.
|
||||||
|
References
Anon. Adefovir. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 09/09/2015. Buckingham R (ed). Adefovir. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 09/09/2015. Dipivoxil Tablet (Sigmapharm Laboratories, LLC). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 09/09/2015. McEvoy GK, Snow EK, Miller J et al (eds). Adefovir Dipivoxil. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 09/09/2015.
|