Allergic conditions
Adult: 8 mg, as necessary, up to tid.
Child: ≥12 yr Same as adult dose.
Child: ≥12 yr Same as adult dose.
|
Indications and Dosage
Oral
Allergic conditions Adult: 8 mg, as necessary, up to tid.
Child: ≥12 yr Same as adult dose. |
|
Renal Impairment
Severe: Contraindicated.
|
|
Administration
May be taken with or without food.
|
|
Contraindications
Severe renal impairment.
|
|
Special Precautions
Patient w/ porphyria, epilepsy. Renal impairment. Elderly. Pregnancy and lactation.
|
|
Adverse Reactions
Anticholinergic effects, GI disturbance, headache, psychomotor impairment, somnolence, dry mouth, rash, blurred vision, urinary retention. Rarely, dizziness, dyspnoea, angioedema.
|
|
PO: B (FDA Pregnancy Category B for acrivastine/pseudoephedrine hydrochloride combination.)
|
|
Patient Counseling Information
This drug may cause dizziness and somnolence, if affected, do not drive or operate machinery.
|
|
Drug Interactions
May produce additional mental depression w/ CNS depressants.
|
|
Food Interaction
May produce additional mental depression w/ alcohol.
|
|
Action
Description: Acrivastine, an alkylamine, is a second generation antihistamine structurally related to triprolidine. It is a competitive antagonist of H1-receptors but lacks anticholinergic effects.
Onset: 15 min. Pharmacokinetics: Absorption: Rapidly absorbed from the GI tract. Time to peak plasma concentration: Approx 1.5 hr. Distribution: Volume of distribution: Approx 0.5-0.8 L/kg. Plasma protein binding: Approx 50%, mainly to albumin. Metabolism: Minimally metabolised in the liver into its active metabolite, propionic acid. Excretion: Via urine (84%) and faeces (13%). Elimination half-life: Approx 2-4 hr. |
|
Chemical Structure
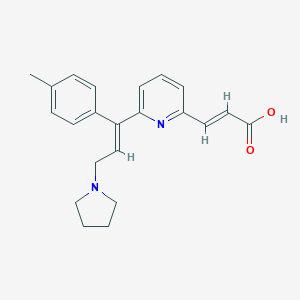 Source: National Center for Biotechnology Information. PubChem Database. Acrivastine, CID=5284514, https://pubchem.ncbi.nlm.nih.gov/compound/Acrivastine (accessed on Jan. 20, 2020) |
|
Storage
Store at or below 30°C.
|
|
MIMS Class
|
|
ATC Classification
R06AX18 - acrivastine ; Belongs to the class of other antihistamines for systemic use.
|
|
References
Anon. Acrivastine and Pseudoephedrine. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 09/08/2016. Buckingham R (ed). Acrivastine. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 09/08/2016. Joint Formulary Committee. Acrivastine. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 09/08/2016. McEvoy GK, Snow EK, Miller J et al (eds). Acrivastine. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 09/08/2016.
|